Abstract
A P2-layered oxide using copper as the active redox metal has been discovered. It has a composition of Na⅔Cu⅓Mn⅔O2, and can withstand a thousand cycles, maintaining 61% of its original capacity. We demonstrate that copper can enable not only high voltage, but also excellent stability. This work opens up a new avenue of oxide design for high energy, cost effective battery systems.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
Energy storage has become an ubiquitous part of our present society. This will become more important as our energy and transportation infrastructure continues undergoing its transformation to becoming fully sustainable and renewable. Batteries are the cornerstone of energy storage, and the development of higher performance, lower cost, and more sustainable materials is a highly researched topic.1 With regard to lithium ion, the dominant high energy battery technology, the move away from cobalt-rich materials has been ongoing for some time, as this element is particularly costly and is subject to economic constraints.2 Recently, as an alternative to lithium ion, sodium ion battery technology has been subject to more extensive examination. Indeed prototype pouch cells are under development that demonstrate this technology.3 Most interestingly, sodium ion batteries have enabled the use of both new types of low cost intercalation compounds and previously inaccessible metal redox couples.4–6 The discovery of the reversible Fe3+/Fe4+ couple in the O3 and P2-type layered oxide structures was particularly important in demonstrating this.7–9 Unfortunately, layered P2 Na-containing oxides utilizing the Fe3+/Fe4+ and Mn3+/Mn4+ redox couples suffer from high polarization and quick capacity fade.10 The Fe-containing O3-type is kinetically facile, but limited in the amount of accessible sodium and extremely sensitive to processing conditions.9–11 These inherent issues are not easily addressed. Thus, there is still an urgent need for further advancements in advanced materials for sodium ion batteries.
While developing new materials, it was realized that cathode materials using copper had not been adequately screened. This may be due to the fact that the Cu3+ and Cu4+ oxidation states are relatively uncommon, with KCuO2 and some Cu-containing superconductors being the most well-known. Spinel-type LiCu½Mn1½O4 was developed and reported in 1997 for lithium ion batteries.12,13 It never garnered the intense interest of its nickel analogue, LiNi½Mn1½O4, due to the complex and undesirable mixing of lithium and copper in both tetrahedral and octahedral sites.14 Despite the issues in developing cathode materials based on copper for lithium ion batteries, we have found that the Cu2+/Cu3+ redox couple works very well in P2-type sodium layered oxides. Indeed, another recent report has also come to the same initial conclusion.15 This opens up a new avenue for design of this important class of materials.
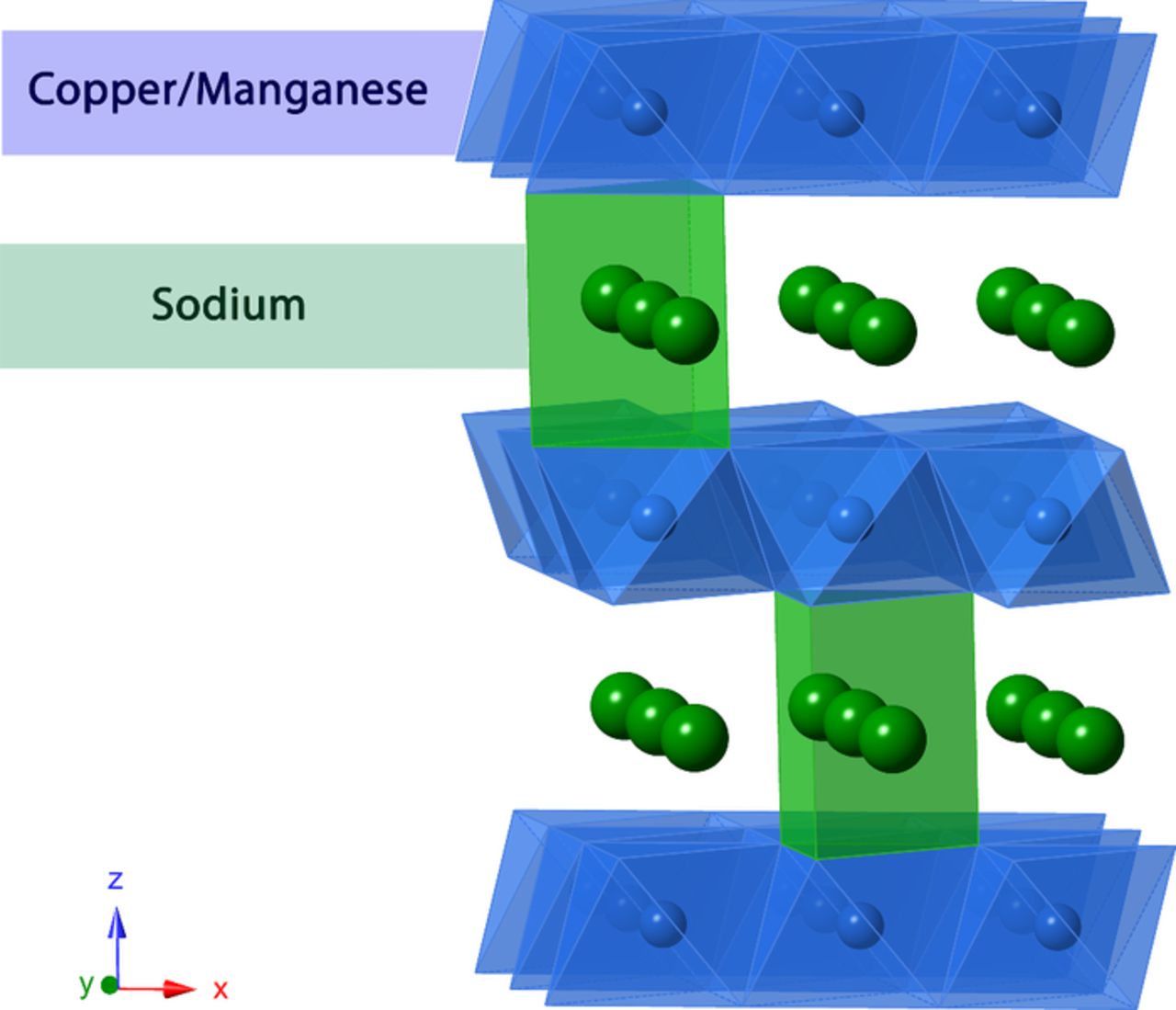
The nomenclature of P2-type oxides with hexagonal crystal structure was proposed by Delmas & Hagenmuller, referring to the ABBA stacking of the transition metal (MO2) layers in each cell and prismatic environment of the sodium ions (Figure 1).16 The O3-type (octahedral alkali ion) is also very common, which encompasses almost all commercial lithium layered oxides, along with a variety of sodium-containing oxides – crystallizing in both the rhombohedral and monoclinic crystal structures. The chemistry is particularly rich for Na-containing P2 and O3 materials, with extensive possibilities for cationic substitution into the transition metal layers.
Figure 1. Crystal structure of P2-type oxide with transition metal layer and prismatic sodium layer in-between.
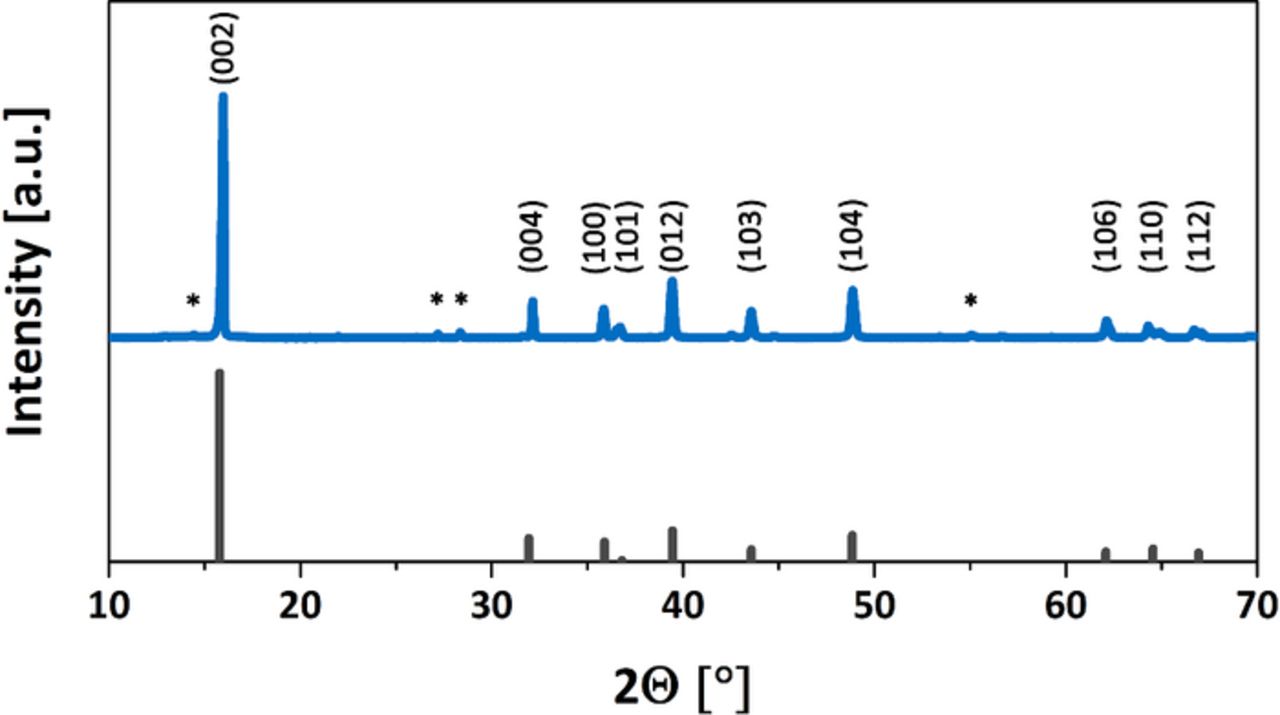
Manganese is the key supporting element in most of these types of oxides, due to its low cost, nontoxicity, useful oxidation states, and structure-promoting properties. Mn3+/Mn4+ can be used effectively as a redox couple, but for practically high energy density, Ni2+/Ni3+/Ni4+ or Fe3+/Fe4+ has been necessary as the active redox couple, with Mn4+ mostly as a spectator.8,17 In particular, advances with the Ni system have been relatively fast, especially with the discovery of the stabilizing effect of lithium in transition metal layers.18–20 Since Na⅔Ni⅓Mn⅔O2 had already been examined as a model P2-type sodium-intercalating oxide system, we replaced Ni2+ with Cu2+, thus building  (see Supplementary Information file for experimental details).17 Powder X-ray diffraction (XRD) revealed that as-synthesized Na⅔Cu⅓Mn⅔O2 was indeed isostructural with the reference hexagonal Ni-containing analogue (Figure 2).
(see Supplementary Information file for experimental details).17 Powder X-ray diffraction (XRD) revealed that as-synthesized Na⅔Cu⅓Mn⅔O2 was indeed isostructural with the reference hexagonal Ni-containing analogue (Figure 2).
Figure 2. Powder XRD pattern of Na⅔Cu⅓Mn⅔O2 and reference peaks of PDF# 00-054-0894. Minor impurities marked with an asterisk.
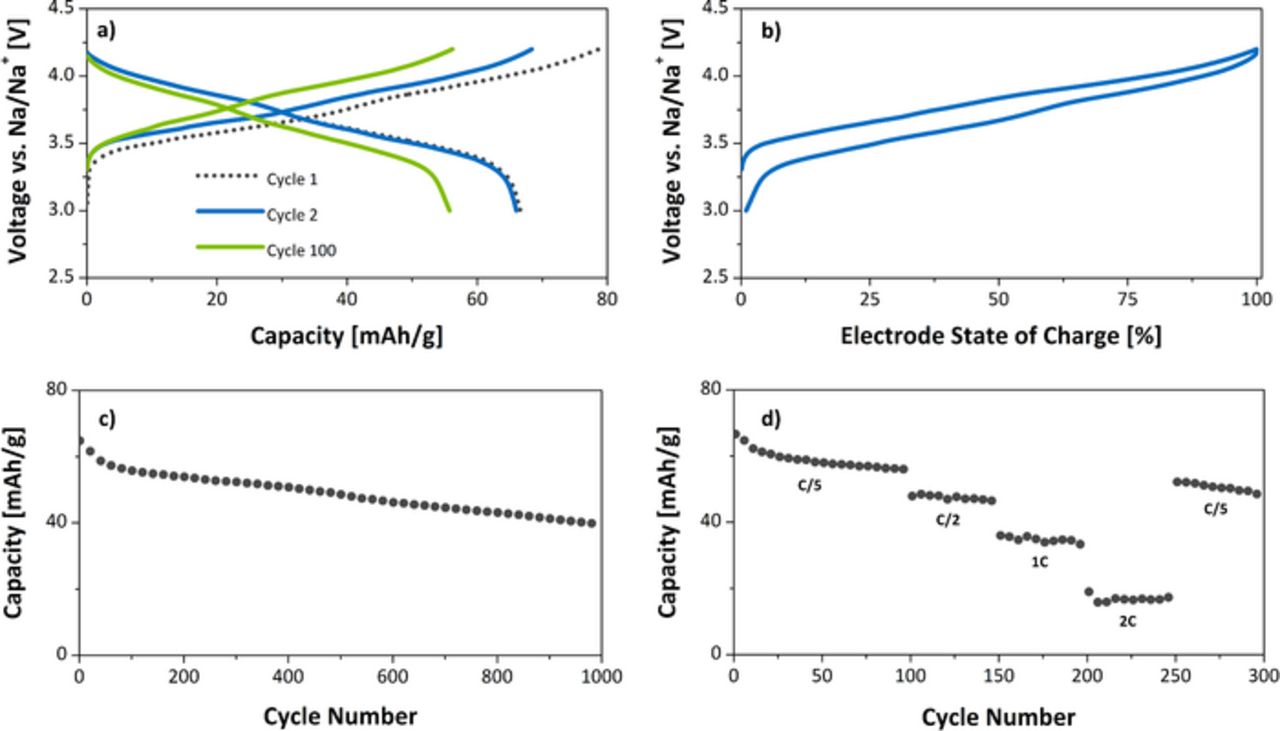
Galvanostatic tests at C/5 in sodium ion half cells (Figure 3a) demonstrate the extraction of almost ⅓Na per formula unit on charge, corresponding to the oxidation of Cu2+ fully to Cu3+, with a gently sloping charge/discharge profile. X-ray absorption spectroscopy (XAS) experiments supported the assertion of the electrochemical oxidation of Cu2+ fully to Cu3+, as discussed in further detail in the next section. This provided 79 mAh/g of charge capacity. On discharge, 67 mAh/g of that capacity was recovered. The charge and discharge capacities began to equilibrate thereafter, with coloumbic efficiency trending to about 98.7%. Figure 3b shows the second cycle with respect to the state of charge (67 mAh/g) to illustrate the low overpotentials observed in Na⅔Cu⅓Mn⅔O2. At C/5, an average of 70 mV of overpotential was calculated over the course of an entire charge/discharge cycle, which is an average difference of 140 mV from charge to discharge. As seen, this effect is less pronounced at high voltage and more so at low voltage. Overall, this property is especially impressive considering the moderate mass loadings and electrode additives used. Nano-structuring, which reduces volumetric energy density is also completely unnecessary, as the large micron-sized particles already perform well (see Supplementary Information file).21 The most dramatic capacity fade of the material was seen in the first 40 cycles, and thereafter showed a constant fade of about 0.1% per cycle. Figure 3c illustrates this at a rate of C/4 up to one-thousand cycles. 61% of the capacity was retained during this test, which is among the best observed for layered oxides for sodium ion batteries.
Figure 3. a) Galvanostatic charge/discharge profiles of Na/Na⅔Cu⅓Mn⅔O2 cell b) Second cycle charge/discharge curves illustrating overpotentials at various states of charge c) Discharge capacity vs. cycle number at C/4 d) Rate capability results.
Rate tests further support this in Figure 3d by displaying very low amounts of capacity depression until about 2C. At this point about 16 mAh/g of capacity is obtained. On returning to C/5 rate, capacity is recovered, correlating well with the long term cycling. Mindful of potential day-to-day energy storage applications of this material, these tests show the good capabilities of a copper-containing P2 oxide – sustained long-term cycling and periodic high rate intermittent charging/discharging.
X-ray absorption spectroscopy (XAS) is a useful technique to determine the variations in local and electronic structure near the absorbing atoms in an elemental-selective way. This can be effectively used to discover the element responsible for the electrochemical activity in a system. In general, the changes in edge energy are correlated to the variations in the average oxidation state of the absorbing atoms being analyzed.22,23
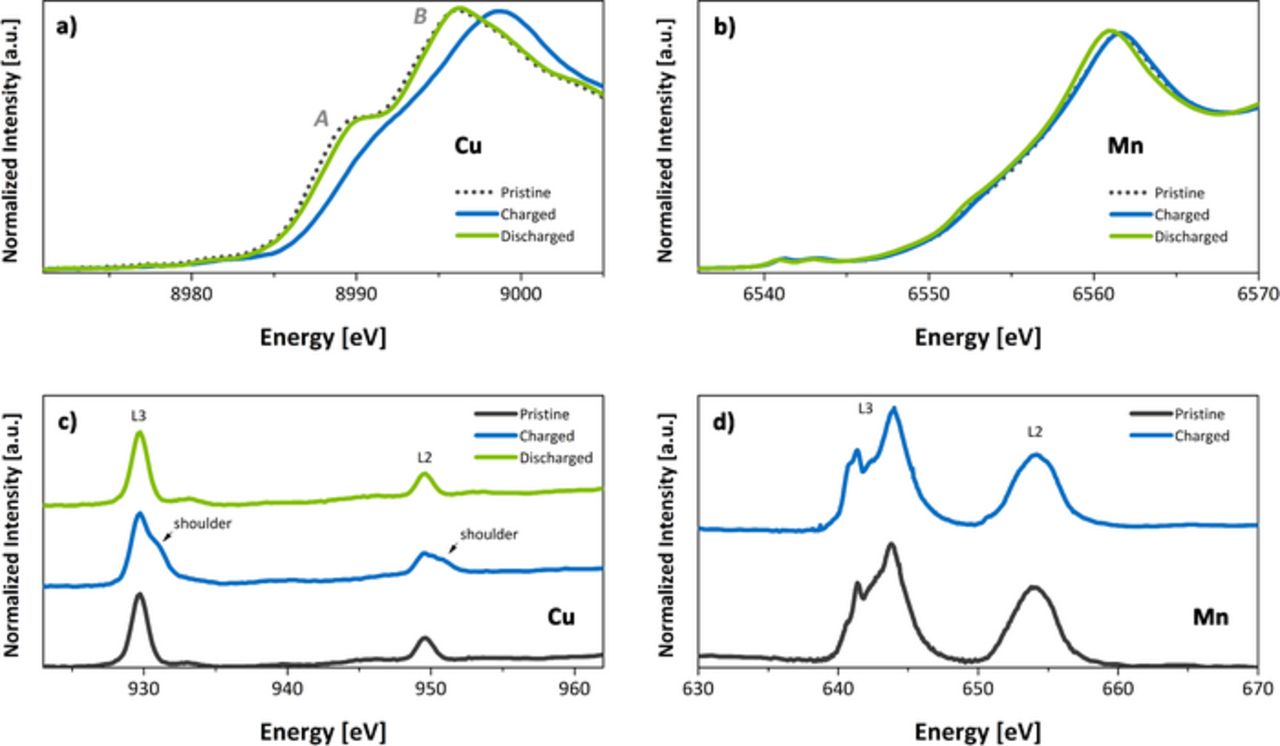
Figure 4a displays the Cu K-edge X-ray absorption near edge structure (XANES) data. Charged cells were brought to about 4.2 V vs. Na/Na+, while discharged to 3.0 V. Pristine samples exhibit a set of key features labelled as A and B in the first figure. These features are linked to the electric dipole transitions from the 1s state to different np final states. Specifically, A represents the ls state to the axial 4p states and B to the planar 4p states.24 In fully charged electrodes, the edge shifts by approximately 2 eV to indicate the Cu2+ to Cu3+ transition.25 During discharge, the Cu K-edge shifts reversibly to lower energies, reaching a position identical to that observed for the pristine material. This demonstrates that copper ions are reduced back to the Cu2+ oxidation state during discharge. Figure 4b shows the Mn K-edge XANES spectra. Unlike the Cu K-edge, negligible edge shift is observed during sodium intercalation/de-intercalation process, suggesting the absence of significant Mn redox activities within the bulk of the particle.
Figure 4. X-ray Absorption Spectra of a) Copper K-edge b) Manganese K-edge c) Copper L-edge d) Manganese L-edge.
Mn and Cu L-edge XAS experiments (probing the transition from metal 2p orbitals to unoccupied 3d orbitals) were performed in the fluorescence yield (FY) mode. Here, the typical probing depth is about 50 nm compared to the K-edge XANES experiments, which probe the bulk. Such a comprehensive combination of soft and hard XAS is important and usually necessary because of the potential gradient charge compensation mechanisms across the battery particles.26–28 One can determine from the XAS/FY spectra that Mn mostly remained in an oxidation state of Mn4+ before and after the charging.28 This observation is consistent with the Mn K-edge XANES results (Figure 4b), indicating that Mn is a charge spectator (electrochemically inactive) in the charging process. In contrast, the Cu L-edge XAS spectra clearly show that Cu was oxidized during the charging process, as evidenced by the increased intensity of the high-energy shoulders in the absorption edges (Figure 4c, both L3 and L2 edge).29 The Cu oxidation state was then reduced to Cu2+ in the discharged state. This is consistent with the data from the hard XANES experiments.
The prospective energy density gains that can be achieved with P2-type oxides utilizing copper were calculated. Assuming a theoretical hard carbon anode with 300 mAh/g of capacity at 0.3 V vs. Na/Na+, the Cu-containing P2-types oxides can provide about 200 Wh/kg of energy density, despite cycling less than ⅓Na. Based on our data, we believe the ability to cycle ½Na will be further possible with or without small amounts of other metal substitutes, achieving over 300 Wh/kg of energy density, on par with the best nickel or cobalt-containing variants.30 This is an exciting prospect for researchers developing next generation low cost battery systems.
We conclusively report on the advantages of using copper as a redox active element in P2-type cathode materials. First, copper has a slightly higher voltage than nickel in the P2 system. Second, the stability of the system using normal, micron-sized particles and good electrode mass loadings is excellent, demonstrating over 1000 cycles, an achievement not usually observed for sodium ion electrodes. Furthermore, there is room for optimization of the stoichiometry of the metals, as has been done with nickel in literature.17–19,30,31 Elements, such as iron, aluminum, magnesium, titanium, and lithium could prove to be the most useful substitutes for copper and manganese to enable enhanced capabilities, from both cost and performance standpoint. We encourage the community to continue to uncover both the electronic and structural properties of this and related materials. With these findings, sodium ion battery chemistry has been moved one large step closer to important energy storage applications.
Acknowledgments
The present work was supported by the Singapore National Research Foundation (NRF) through its Campus for Research Excellence and Technological Enterprise (CREATE) program. The authors acknowledge the support of Stanford Synchrotron Radiation Lightsource, a Directorate of SLAC National Accelerator Laboratory and an Office of Science User Facility operated for the U.S. Department of Energy Office of Science by Stanford University. Guidance and support from Dr. Guoying Chen and Dr. Marca Doeff at LBNL is greatly appreciated. We thank Arun Nagasubramanian, Steffen Schluter, Shahnaz Ghasemi, Heryani Ahmad, Irina Gocheva, and Denis Yu for discussions and support. We also thank Dr. Ryan Davis for assisting with the synchrotron experiments.