Abstract
Outstanding characteristics of graphene are its high thermal conductivity, inherent high capacity, extremely large specific surface area, high strength, ductility, and remarkable chemical inertness, making it an attractive candidate in the corrosion barrier field. Since graphene coating does not change the thickness and appearance of the substrate, it is an ideal coating for protecting a metal substrate from destructive effects. Between various deposition procedures of graphene coatings on metal surfaces, i.e., electrophoretic deposition, dip coating, spray coating, spin coating, etc., chemical vapour deposition (CVD)-grown graphene coatings have been shown to improve the corrosion resistance of graphene-coated metals significantly. This review is focused on the protective properties of graphene coatings deposited by CVD on different metal substrates and exposed to corrosive environments.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Protecting metals from oxidation and corrosion is a controversial topic that has been the subject of various research for decades and is of great importance for various industrial and academic applications 1 New materials and approaches were proposed to protect the metals in a corrosive environment 2 As time goes by, the approaches become more practical, and the coatings and materials for metal protection become more robust and reliable. A desirable coating does not affect the dimensions, appearance, optical properties, and even the thermal and electrical conductivity of the coated metal. The introduction of new types of nanomaterials (i.e., two-dimensional (2D) nanomaterials) opened a vast opportunity to explore these materials for various applications, i.e., corrosion and oxidation protection barriers 3 Among the 2D materials, graphene was the first isolated 2D material and exhibited excellent chemical inertness, high electrical conductivity, optical transparency, good thermal stability (higher than 1500 °C under an inert environment), high mechanical stability, and water and gas impermeability (thanks to its sp 2 orbital hybridization that forms a natural diffusion barrier). 4–8 Among the different techniques of producing graphene, i.e., mechanical exfoliation of graphite, 9 epitaxial growth of graphene on silicon carbide (SiC), 10 reduction of graphene oxide, 11–13 unzipping carbon nanotubes (CNTs) 14 and chemical vapour deposition (CVD), 15,16 due to scalability and cost-effective procedure, CVD emerged as the most popular method for producing graphene on various metal and dielectric substrates. 17,18 As a one-atom-thick material, graphene presented an outstanding ability for protecting metals from oxidation and corrosion in destructive environments. Therefore, many researchers reported the application of graphene as corrosion protective coatings on various metal substrates 13,16,19–44 and as filler for enhancing the protective properties of polymer coatings. 33,34,45–50
In the CVD method, the formation of graphene islands on the metal catalyst surface is the first step of the growth process. The nucleation of graphene in CVD is affected by the following factors: growth temperature, catalyst affinity to carbon, carbon source, and substrate quality. 7 The presence of oxides or other contaminants on the metal surface significantly influences the graphene nucleation mechanism. 51 Many studies 21,28,52 have demonstrated that single and several layer graphene coatings grown by CVD can protect different underlying substrates from oxidation under ambient conditions, 24 hydrogen peroxide wet corrosion, 53 and corrosion in different electrolytes like sulphate and chloride solutions. 20,21,26,31,33,52 Some authors have suggested that the structural defects and wrinkles in the conducting graphene coating can further promote local oxidation of the protected material, mainly at the grain boundaries. Alternatively, the increased temperature during graphene growth can also change the properties of the underlying metal substrate. 23,24,32,52
Furthermore, some factors accelerate the corrosion rate of the metal substrate coated by graphene layer: the presence of defects and disorders in the graphene coating, 21,35,54–56 the thickness of the graphene coating (thinner coating presented higher corrosion rate compared to a thicker coating), 21,26,57 and the amount of graphene filler (percolation threshold) and the homogeneity of the dispersed graphene in the case of the composite coatings. 19,46–50,58,59 Furthermore, doping graphene with N and F elements 27,60 was reported as a practical approach for enhancing the corrosion barrier ability of the graphene coating.
Here we review the reports provided by various research teams regarding the corrosion protection of CVD-grown graphene on various metal substrates, and the impact of the dopants on the corrosion protection properties of the graphene coating. Although recent published review articles have already provided significant information about the corrosion stability of graphene coatings and its characteristics, 61–67 they did not challenge a specific graphene synthesis technique. In contrast, our review article focused on applying CVD-grown graphene as a corrosion barrier and the challenges facing such coatings.
Graphene Coatings on Copper, Nickel, Platinum, and Stainless Steel
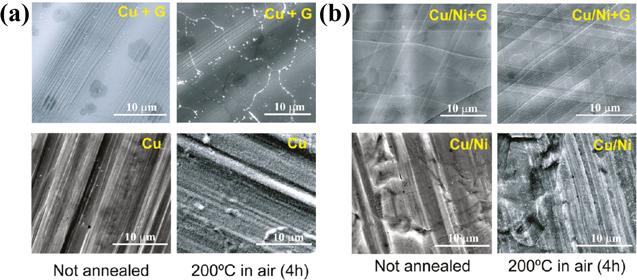
Chen et al. were the first to demonstrate the ability of CVD-grown graphene to protect the surface of copper (Cu) and Cu/nickel (Ni) metallic substrates from air oxidation as a passivation layer, and graphene also acted as an effective barrier against hydrogen peroxide (H2O2). 53 They observed that metal oxidation occurred at graphene grain boundaries, while within grains, graphene sheets provide near-perfect protection (Fig. 1), which then also was observed by other research teams. 21,53,54,68 Furthermore, graphene could successfully protect the metal surface from corrosion after heating at 200 °C for over 4 h in ambient conditions.
Figure 1. (a) SEM images of graphene coated (upper) and uncoated (lower) Cu foil (b) SEM images of graphene coated (upper) and uncoated (lower) Cu/Ni foil, before (left column) and after (right column) annealing in air (Reprinted from Ref. 53 Copyright (2011), with permission from American Chemical Society).
Download figure:
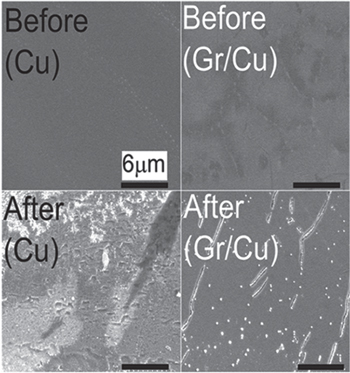
Standard image High-resolution imageProtecting Cu and Ni from corrosion in an 0.1 M aerated sodium sulfate (Na2SO4) solution was investigated using electrochemical tests to show the impact of a few layers and multilayer graphene on protecting metal substrates from corrosion. 21 The electrochemical test showed that graphene reduced metal oxidation and oxygen reduction, and the graphene-coated Cu was corroded in the aerated Na2SO4 solution seven times slower than bare Cu (Fig.2). Moreover, this report showed that Ni coated with multilayer graphene was corroded much slower than Ni coated with 4-layer graphene.
Figure 2. SEM images of Cu and Gr/Cu sample before and after exposure to 0.1 M aerated Na2SO4 solution (Reprinted from Ref. 21 Copyright (2012), with permission from American Chemical Society).
Download figure:
Standard image High-resolution imageReducing the electrochemical corrosion rate of Cu and Ni in aqueous media by using graphene as a corrosion barrier layer was reported by several teams. 20–26 In all cases, the deposited graphene layers proved capable of enhancing corrosion protection of pure metals, however, primarily slowing the anodic dissolution reactions for Ni and the cathodic reduction reactions for Cu. 20 To study the corrosion protection properties of graphene on platinum (Pt) substrate, CVD-grown graphene on Pt(100) was exposed to different experimental conditions: (a) ambient temperature and pressure for six months and for 75 min at 60 °C, (b) 14 h to Milli-Q water at ambient condition and 75 min at 60 °C, and (c) 75 min in NaCl solution at room temperature and for 75 min at 60 °C. 41 Investigating the changes to the surface structure and chemistry showed that the graphene layer was intact in all conditions, even if the underlying Pt surface was oxidized, except in NaCl solution at 60 °C. This phenomenon suggested that the oxidizing agents diffused beneath the graphene layer while the graphene layer stayed intact.
Furthermore, some studies revealed that the thickness of the graphene layer affected its corrosion protection ability, and the thicker ones protected the metal substrates more effectively. 21,26 Impermeability of graphene to gases and liquids was introduced as another factor that provides graphene's oxidation and corrosion protection ability. 4 Raman et al. reported that the ability of graphene film to act as an impermeable ionic barrier was the reason for significantly improved corrosion resistance of a Cu substrate. 22
Its thermal stability at high temperatures motivated researchers to apply graphene as a coating on metals to protect them from oxidation at high temperatures. Different research groups reported the application of graphene as an oxidation barrier coating for various substrates, i.e., Cu, Ni, silicon, molybdenum (Mo). 23,30,68,69 Kousalya et al. showed that graphene coating was stable under vigorous flow boiling conditions and successfully used as an oxidation barrier coating for Cu, even under long-term exposure to the oxidation environment. 68
Schriver et al. reported the same results for the effectiveness of CVD-grown graphene as an oxidation barrier for protecting Cu substrate but for short-term oxidation protection. 23 They believed that the thermal stability of graphene and its impermeability to gases was the reason graphene acted as a good oxidation barrier. 4 However, the results indicated the ineffectiveness of graphene coating over a long time of exposure to H2O and O2. Penetrating H2O and O2 to the metal substrate through graphene defects resulted in non-uniform corrosion, cracks, and oxide over the surface and bulk of the substrate. The short-time effectiveness of graphene coating for protecting the metal substrates was also reported by Dong et al. 16 The CVD-grown graphene on Cu substrate improved the Cu corrosion properties in 3.5 wt.% NaCl solution for a short time. However, prolonging the immersion resulted in detaching the graphene film from the Cu substrate, which no longer provided barrier protection 26 and resulted in even more severe corrosion than the bare Cu. They also observed that the graphene-coated Cu was generally corroded, while the bare Cu was corroded locally.
Further studies of graphene as a corrosion barrier revealed that the corrosion was not limited to grain boundaries. 54 Based on the reports provided by Hsieh et al., the limited passivation provided by CVD-grown graphene was due to the nanometre-size defects produced in the graphene coating during the deposition process. Corrosive agents were transported across and parallel to graphene layers and could easily penetrate the underlying metal through these defects. Wlasny et al. investigated the applicability of graphene for protecting Cu substrate from corrosion by atmospheric gases. 24 They showed that the defective sites of graphene resulted in penetration of the atmospheric gases to the substrate, and corrosion of the metal substrate progressed underneath the graphene coating. Therefore, they believed that graphene acted as an attractive corrosion barrier in macroscale and large-scale devices, while the graphene coating was not efficient for nanotechnology.
Although graphene presented a reliable protection behaviour in most cases, some reports showed that graphene coatings fail to protect the underlying metal in galvanic corrosion conditions. 25 Ambrosi et al. observed the detrimental effects of the CVD-grown graphene on Ni once damaged. 25 Based on their observation, the galvanic corrosion damaged graphene dramatically, and the damaged graphene coating had a tremendous detrimental effect on the Ni substrates. The metal substrate of the damaged graphene exhibited a higher corrosion rate even when compared to the unprotected metal. Dong et al. investigated the effect of substrate morphology on the protection ability of graphene coating. 26 Testing of CVD-grown graphene on polished and unpolished Cu substrates showed that graphene failed to protect Cu in prolonged exposure to 3.5 wt.% NaCl solution. Moreover, in short exposure, graphene provided better protection for the polished Cu because of the large grain size and fewer grain boundaries of graphene coating on polished Cu. After prolonged exposure, the graphene was detached more easily from the polished Cu than the unpolished Cu, which showed that graphene could not protect Cu in long-term exposure to ionic solutions.
To modify the protective ability of graphene on metal substrates, Kumar et al. used nitrogen (N)-doped graphene as a protective coating on stainless steel (SS) in a 3.5% NaCl solution. 27 They reported that the graphene coating on SS provided a robust corrosion resistance and protected the SS in a 3.5% NaCl solution successfully. The underlying mechanism of the better protection of N-doped graphene was related to the adsorption energies of graphene coating on SS substrate. Molecular dynamics simulation showed that the N-doped graphene was stably chemisorbed on SS, while the graphene coating was adsorbed in a parallel orientation on the SS surface. N-doped graphene on Cu substrate also presented a long-term protection ability of the substrate in ambient conditions. 70 The N-doped graphene reduced the natural oxidization of the Cu substrate in long-term exposure and increasing the N-dopant concentration positively impacted this phenomenon. Reducing the conductivity of N-doped graphene coating compared to pristine graphene was suggested for the better protection ability of N-doped graphene coating. In the long-term exposure, reducing the electrical conductivity of the coating prevented the electrochemical reaction between the N-doped graphene coating and Cu substrate, therefore inhibiting the galvanic corrosion by cutting electron transport across the interface of the coating. Doping graphene with fluorine (F) was also reported as an effective approach for prolonging the graphene protection ability of the substrate from corrosion in 3.5 wt% NaCl solution. 60 The electrochemical tests showed that compared with the pristine graphene, the low-frequency impedance modulus of F-doped graphene was an order of magnitude higher. The better corrosion protection strategy of F-doped graphene was due to the bonding of F atoms with carbon elements on the edges of vacancy defects, which inhibited the corrosion-related molecules passed through the film via intrinsic defects. 54
Most of the reports showed the graphene weakness for protecting metal substrates against corrosion and oxidation for long-term exposure to a corrosive environment. In contrast, some reports show that graphene can protect the substrate in specific conditions after being exposed to a harsh environment for a long time. Stoot et al. reported the application of CVD-grown multilayer graphene for protecting SS with Ni seed layer (G/Ni/SS). 31 Based on their findings, a long-term exposure (three weeks) of the samples, SS with only a Ni seed layer (Ni/SS) and G/Ni/SS to boiling seawater, revealed a clear superiority of the G/Ni/SS compared to Ni/SS. Furthermore, after the test, the defect density of graphene coating was unchanged, and the coating was intact.
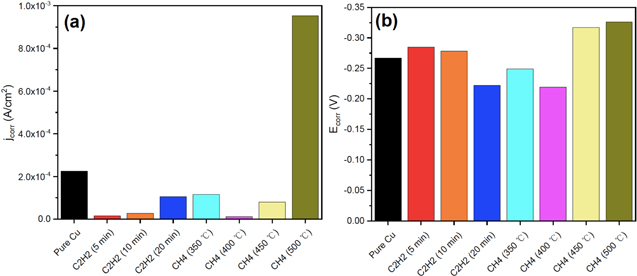
Defects and disorders were introduced as factors that provide a site for penetration of corrosive species underneath graphene coatings that led to oxidizing the metal substrates. Therefore, modifying the graphene deposition technique for producing high quality, low defect graphene seems an essential step in this regard. In order to produce high-quality CVD-grown graphene, using ethanol as a gas precursor was suggested by Ji et al. 71 A bi-layer graphene was produced on Cu substrate that reduced the corrosion rate of the substrate 24 times compared to the bare Cu. Moreover, the stability of the corrosion protection behaviour of the graphene coating was investigated by storing the coated sample for one year in the ambient environment, which indicated that the graphene coating could preserve its barrier properties over time. To improve the quality of CVD-grown graphene, Huang et al. investigated the impact of various deposition parameters on the quality and barrier properties of the deposited graphene on Cu at temperatures lower than 600 °C. 72 They investigated different gas precursors, different deposition temperatures, and different deposition times. Their result showed the importance of an optimized technique on the uniformity, crystallinity, and coverage of the deposited graphene (Fig. 3).
Figure 3. (a) jcorr and (b) Ecorr of bare Cu and graphene-coated Cu using different growth conditions (Reprinted from Ref. 72 Copyright (2018), with permission from MDPI).
Download figure:
Standard image High-resolution imageNot only the quality of the produced graphene coating is effective on the barrier properties of graphene, the crystal structure and quality of the underlying substrates reported as effective factors for the performance of the graphene coating. Wlasny et al. investigated the impact of the crystal structure of the Cu substrate on the protection ability of graphene coating. 32 Mono and polycrystal Cu substrates were coated with graphene and exposed to urban atmospheric conditions for a short time. The results indicated no trace of copper oxide on the monocrystal Cu, while an oxide layer was developed on the polycrystal Cu due to the electrolyte intercalation at the graphene/Cu interface and the high corrosion rate.
Caesium-Doped Graphene Coatings on Copper
The CVD caesium-doped graphene coating on Cu foil (G/Cs-Cu) was obtained from Cs2CO3 (as Cs precursor) at 1000 °C in CH4/H2/Ar gas mixture. 73 Since it was shown that heating Cs2CO3 up to 600 °C results in its decomposition, 74 the presence of Cs2CO3 during the graphene growth resulted in the doping of the graphene layer with Cs. The pure graphene coating on Cu foil (G-Cu) was grown in a similar synthesis procedure without introducing the Cs2CO3.
Compared to pure graphene (C1s at 284.5 eV), the X-ray photoelectron spectroscopy (XPS) spectra of the G/Cs coating revealed a shift of the C1s to higher binding energies (285.1 eV). Furthermore, Cs3d5/2 peaks, which appeared in the XPS spectra due to the Cs doping, shifted to lower binding energies (724.4 eV) than the Cs3d5/2 normal peak position (726.4 eV). These shifts imply the n-type character of the doping and charge transfer between Cs and graphene. 73 Raman results proved that pure graphene coating comprises fewer layers, fewer defects, and a larger domain size than the G/Cs coating. Ultraviolet photoelectron spectroscopy (UPS) indicated that Cs-doped graphene reduced the work function of graphene by 1.2 eV.
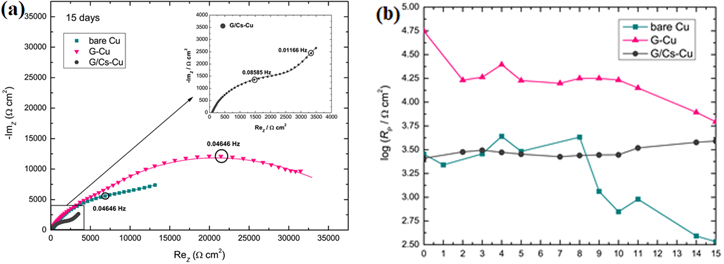
The electrochemical impedance spectroscopy (EIS) revealed that the impedance values (Fig. 4a) of G/Cs-Cu coating were significantly lower than of G-Cu coating during immersion in 0.1 M NaCl (60). Due to lower adhesion of Cs-doped graphene coating and decreased conductivity of graphene after doping with alkali metals, 75,76 promoted the active dissolution of Cu. In addition, reduced electron work function could cause the Cu surface to become more electrochemically active. 77 The obtained EIS spectra were fitted with an equivalent electrical circuit (EEC) consisting of coating pore resistance, Rp, constant phase element, CPEc, corresponding to coating capacitance, charge-transfer resistance, Rct, and constant phase element, CPEdl, corresponding to the electrochemical double layer on the metal/electrolyte interface. The G-Cu sample exhibited the highest values of coating pore resistance (Fig. 4b), pointing to superior corrosion stability of the graphene-coated copper in respect to Cs-doped graphene coating.
Figure 4. (a) Comparison of impedance spectra for bare Cu, G-Cu and G/Cs-Cu after 15 days of immersion in 0.1M NaCl, and (b) time dependences of Rp for bare Cu, G-Cu and G/Cs-Cu, during 15 days of immersion in 0.1 M NaCl (Reprinted from Ref. 73 Copyright (2020), with permission from Elsevier).
Download figure:
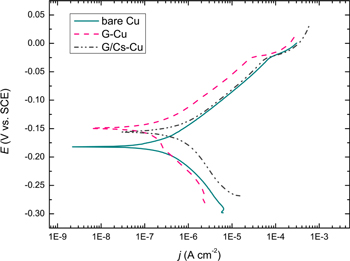
Standard image High-resolution imageThe linear Tafel segments of the anodic and cathodic potentiodynamic polarization curves (Fig. 5) were extrapolated to corrosion potential to obtain the corrosion current density, jcorr, while ba and bc are anodic and cathodic Tafel slopes, and Rpol is the polarization resistance. The G-Cu coating exhibited the lowest corrosion current density values (0.11 μA cm−2), while G/Cs-Cu exhibited almost three times higher jcorr (0.65 μA cm−2) compared to bare Cu (0.27 μA cm−2). Similarly, the polarization resistance, Rpol, the value was much higher for G-Cu (43.1 kΩ cm2) compared to bare Cu (25.2 kΩ cm2), whereas G/Cs-Cu exhibited the lowest Rpol values (17.1 kΩ cm2). The corrosion rates, vcorr, calculated from the obtained jcorr values, according to the Eq. 1, where M(Cu) is the molar mass of copper, ρ(Cu) is copper density, z is the number of electrons exchanged in the copper corrosion reaction, and F is Faraday's constant, were calculated to be 6.26 nm yr−1 for bare Cu, 2.55 nm yr−1 for G-Cu, and 15.1 nm yr−1 for G/Cs-Cu. 73
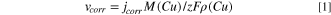
Figure 5. Polarization curves of bare Cu, G-Cu and G/Cs-Cu after 15 days of immersion in 0.1M NaCl (Reprinted from Ref. 73 Copyright (2020), with permission from Elsevier).
Download figure:
Standard image High-resolution imageSuch low protective properties of G/Cs-Cu, compared to bare Cu and especially G-Cu, proved by EIS and potentiodynamic sweep (PDS), resulted from Cs doping, which caused Gr to become an n-type doped semiconductor, 78 and therefore could have increased its electrical conductivity. However, as discussed above, there is some evidence that was doping graphene with alkali metals (as opposed to, e.g., nitrogen doping 79 ) could actually decrease its conductivity. 75,76 In addition, the G/Cs coating had a smaller grain size, more disorder and defects than pure graphene and lower work function. 73 These effects taken together could lead to a higher corrosion rate and lower corrosion resistance of the Cs-doped graphene coating compared to the pure graphene one, even the bare copper itself.
Graphene Coatings on Molybdenum
Early studies illustrated that Mo might act as an effective catalyst for carbon nanotubes (CNTs), while iron-Mo could act as a very efficient catalyst for synthesizing both single-walled and multi-walled CNTs with the CVD method. 80,81 However, the use of Mo as a catalyst to produce graphene is still a new challenge. Wu et al. reported the synthesis of large-area graphene on Mo foils using the CVD method. 82 They reported the cooling rate as key to controlling the number of graphene layers and growth rate. Grachova et al. reported the CVD of graphene on sputtered thin films of Mo. 51 Hsieh et al. studied the effect of the addition of Mo to the growth rate of graphene on Cu. 83 Mo affected the graphene quality and increased the growth rate of graphene. The high melting point, low thermal expansion, and low surface roughness of Mo created excellent conditions for synthesizing a large area and high-quality graphene. 51
Various authors considered Mo to passivate spontaneously at the open circuit potential in different aqueous solutions by forming a chemisorbed oxygen layer or a thin layer of Mo mixed (III, IV) valence oxide compounds. 84–89 In acidic solutions, the passive film contained MoO2 with MoO3 and MoO(OH)2, whereas MoO3 and Mo(OH)3 were identified in the alkaline solutions. The electrochemical behaviour of Mo is complex due to the formation of different oxidation state species, both in the film on the metal surface and in the aqueous solution. 90 At anodic overpotentials, transpassive dissolution of Mo occurs, resulting in the growth of a conductive layer of a non-stoichiometric Mo oxide depending on the applied potential. 86,91 The passive film formed on Mo was pH-dependent, and the stability of the passive film decreased with the increasing pH of the solution due to the formation of the soluble products like HMoO4 −and MoO4 2−.
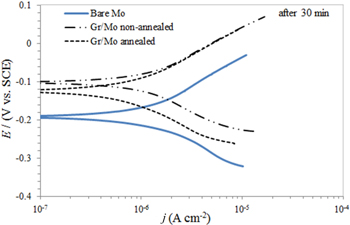
In order to investigate the effect of the annealing process on corrosion behaviour of graphene coatings, the CVD graphene coatings obtained on Mo foils with CH4 as the carbon precursor at 1000 °C in CH4/H2/Ar gas mixture were annealed at different time intervals. 92 Polarization curves of bare and graphene-coated Mo in 0.1 M NaCl (Fig. 6) have shown that graphene coatings on both the non-annealed and annealed Mo shifted the Ecorr towards more positive values with respect to bare Mo, indicating that both graphene coated Mo are less suspectable to corrosion. A relatively constant value of the anodic Tafel slope (close to 150 mV dec−1) for both bare and graphene-coated Mo implied that the mechanism of anodic reaction did not change in the presence of the graphene coating. The obtained values for Ecorr (−190 mV), jcorr (0.96 μA cm−2) and vcorr (0.0049 mm yr−1) for bare Mo after 30 min in 0.1 M NaCl 92 agreed with the previously reported literature data. 93,94 The jcorr values after the initial time of exposure (30 min) confirmed that the graphene coatings on both annealed Mo (0.69 μA cm−2) and non-annealed Mo (0.89 μA cm−2) provided better protection against corrosion compared to bare Mo (0.96 μA cm−2), while the graphene coating on annealed Mo exhibited a lower value of jcorr than the graphene coating on non-annealed Mo. After prolonged exposure (30 days), the calculated corrosion rate for both graphene coatings were 0.018 mm yr−1 for non-annealed Mo and 0.0195 mm yr−1 for annealed Mo compared to bare Mo (0.043 mm yr−1), meaning that graphene coatings on both Mo samples acted as a corrosion barrier for the Mo substrate.
Figure 6. Potentiodynamic plots showing bare and graphene coated Mo after 30 min of exposure to 0.1 M NaCl (Reprinted from Ref. 92 Copyright (2016), with permission from Elsevier).
Download figure:
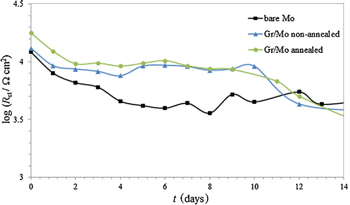
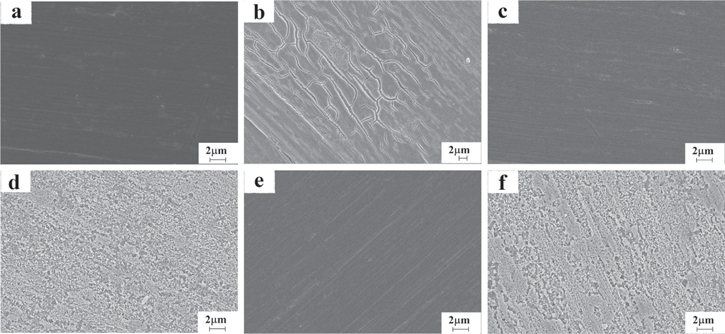
Standard image High-resolution imageEIS measurements have shown that the Rct values of bare Mo were less than the Rct values of both non-annealed and annealed graphene-coated Mo (Fig. 7), indicating the protective properties of the graphene coating. In addition, the greater values of Rct for the graphene coating on annealed Mo compared to that on non-annealed Mo were observed during the initial period of exposure to 0.1 M NaCl because the XRD, XPS and Raman spectroscopy revealed a decreased number of layers and degree of defects in the graphene coatings as the annealing time increased, resulting in larger graphene domains. 92 Consequently, the graphene coating on annealed Mo provided better protection against corrosion during the initial exposure times than the graphene coating on non-annealed Mo, but during prolonged exposure, both graphene coatings demonstrated nearly the same Rct values. The scanning electron micrographs of the bare and graphene coated Mo before and after 10 days of exposure to 0.1 M NaCl (Fig. 8) are in accordance with the results obtained from the electrochemical measurements.
Figure 7. Time dependence of resistance Rct for bare and graphene coated Mo during prolonged exposure to 0.1 M NaCl (Reprinted from Ref.92 Copyright (2016), with permission from Elsevier).
Download figure:
Standard image High-resolution imageFigure 8. SEM images of bare Mo before (a) and after exposure (b), graphene coated non-annealed Mo before (c) and after exposure (d) and graphene coated annealed Mo before (e) and after exposure (f) to 0.1 M NaCl for 10 days (Reprinted from Ref. 92 Copyright (2016), with permission from Elsevier).
Download figure:
Standard image High-resolution imageGraphene Coatings on Platinum-Coated Molybdenum
Pt is one of the known catalysts that, unlike the widely used catalysts (Cu and Ni), is more resistant to oxidation due to its inertness 95 and has a better catalytic ability for decomposition of hydrocarbons and subsequent graphitization than Cu. 96 Unlike Cu, which requires a low-pressure environment to grow graphene, Pt can induce growth of large-grain graphene at ambient pressure, 97 while the weak interaction of Pt substrate with graphene helps reduce the negative effect of the substrate on the quality of graphene. 98,99 Yong et al. reported the growth of uniform bi-layer graphene with giant grain size on Pt thin film. 95 They showed that the sizes of graphene grains depended on the Pt grain size, which was changed by pre-annealing the catalyst. Sun et al. demonstrated the dependence of high-quality multilayer graphene synthesis on proper control over the growth kinetics when using Pt as catalyst. 96
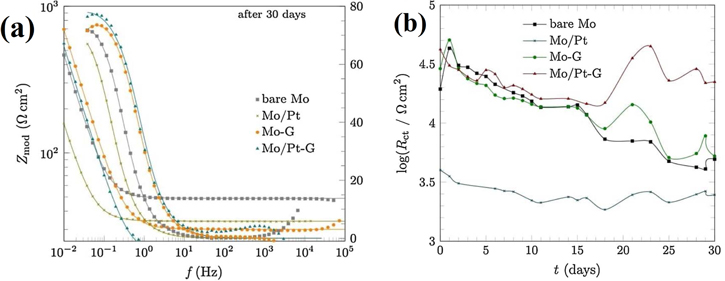
The Bode impedance plots after 30 days of exposure to 0.1 M NaCl (Fig. 9a) depicted higher impedance of graphene coating on Mo foil (Mo-G) and graphene coating on Pt-coated Mo foil (Mo/Pt-G) with respect to both bare Mo and Pt-coated molybdenum (Mo/Pt), while Mo/Pt had the lowest impedance. Mo/Pt-G exhibited the highest charge-transfer resistance values, Rct (Fig. 9b), indicating the role of platinum as a catalyst for CVD synthesis of graphene coating with better protective properties than the graphene coating on bare Mo surface, especially for long-term protection. During the CVD of graphene onto the Mo/Pt surface, some dewetting of the Pt film occurred and left the oxide-free Mo surface inducing better adhesion of graphene coating, as indicated by the lower oxygen content in Mo/Pt-G coating (16.86 at.%) with respect to Mo-G coating (17.81 at.%), obtained from EDS analysis. 100 The lowest jcorr (1.98 μA cm−2) was calculated from the polarization curves for Mo/Pt-G coating compared to Mo-G coating (jcorr 3.20 μA cm−2). The EDS results showed that the Mo/Pt-G contained the smallest amounts of oxygen, indicating the graphene coating completely covered the metal surface. This corresponded to XPS findings that the surface of Mo/Pt-G was less amorphous and less oxidized than that of Mo-G. Moreover, Raman spectroscopy confirmed a thin bi-layer graphene coating with fewer defects and larger domain size on Pt-coated Mo, while the Mo-G was a few-layer with more defects and smaller domain size. 100 The scanning electron micrographs of the bare Mo, Pt-coated Mo, graphene coated Mo, and graphene-coated Mo/Pt before and after 30 days immersion in 0.1 M NaCl (Fig. 10) are in accordance with the results obtained from the electrochemical measurements.
Figure 9. (a) Bode impedance spectra after 30 days of immersion in 0.1 M NaCl, and (b) time dependence of charge-transfer resistance, Rct, for bare Mo, Mo/Pt (Pt-coated Mo foil), Mo-G (graphene-coated Mo foil) and Mo/Pt-G (graphene-coated Mo-Pt), calculated from the EIS spectra obtained for different times of immersion in 0.1 M NaCl (Reprinted from Ref. 100 Copyright (2018), with permission from Elsevier).
Download figure:
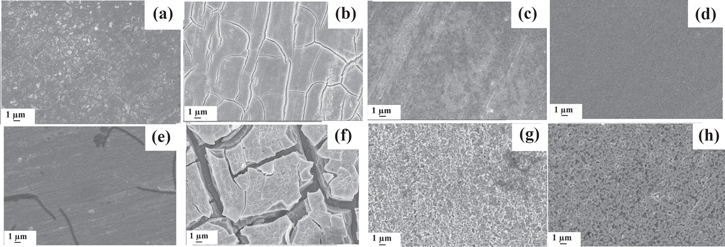
Standard image High-resolution imageFigure 10. FE-SEM images of (a) bare Mo foil, (b) Mo/Pt (Pt-coated Mo foil), (c) Mo-G (graphene coated Mo foil), (d) Mo/Pt-G (graphene-coated Mo/Pt) before immersion in 0.1 M NaCl, and (e) bare Mo, (f) Mo/Pt (Pt-coated Mo foil), (g) Mo-G (graphene-coated Mo foil) and (h) Mo/Pt-G (graphene-coated Mo/Pt) after 30 days immersion in 0.1 M NaCl (Reprinted from Ref. 100 Copyright (2018), with permission from Elsevier).
Download figure:
Standard image High-resolution imageGraphene Coatings on Aluminium
In order to obtain graphene coatings on aluminium, the following procedure was applied: graphene coatings were deposited first on Cu foils at 1000 °C in CH4/H2/Ar gas mixture, 52,101 then were removed from the Cu foils by etching in an aqueous solution of iron nitrate, and finally one, two, three or four graphene layers were transferred successively onto the aluminium surface. 52
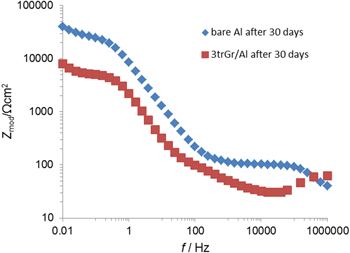
Protective properties of graphene coatings on Al have been tested in 0.1M NaCl using EIS and PDS techniques. Bode impedance plots (Fig. 11) indicated that the graphene coating transferred onto aluminium did not reduce the corrosion rate of aluminium, probably due to weak adhesion of graphene coating, as well as the galvanic corrosion of aluminium that can occur when there is a contact with a more noble metal or another electron conductor with a higher chemical potential than aluminium. 102 Similar results were obtained from PDS measurements: the values of corrosion current density were calculated to be 8.28 μA cm−2 for bare aluminium and 29.1, 38.9, 13.4 and 34.2 μA cm−2 for 1, 2, 3 and 4 transfers for graphene-coated aluminium, respectively. 52 Moreover, the specimen with three graphene layers transferred onto aluminium sequentially had the lowest corrosion rate among the coated specimens but was still higher than bare aluminium.
Figure 11. Bode impedance plots for bare and graphene coated aluminium, after 30 days of exposure to 0.1 M NaCl.
Download figure:
Standard image High-resolution imageTable I summarizes the conditions of CVD technique, characteristics of the deposited graphene layer (mono, bi, few, or multilayers), main finding of each study, and drawbacks regarding the application of the synthesized graphene layer for protecting metal substrates from corrosion and oxidation.
Table I. Overview of the CVD conditions for graphene synthesis and the results of applying graphene as corrosion barrier coating on different substrates.
| CVD conditions | |||||||
|---|---|---|---|---|---|---|---|
| Type of coating | Growth temp. (°C) | Precursor | Pressure | Catalyst/ substrate | Corrosive environment | Results/ Drawbacks | References |
| Monolayer and multilayer | 1040 | CH4 | 500 mTorr | Cu/Si | High temperature oxidation | Better protection of multilayer in long term. Susceptibility of grain boundaries to oxidative reaction | 53 |
| 1000 | 8 Torr | Cu/Ni alloy | H2O2 solution | Short term protection. Penetration of solution underneath graphene in long-term | |||
| Multilayer | 1000 | CH4 | 250 mTorr | Cu | 0.1 M Na2SO4 solution | Graphene coating slowing the corrosion rate. Transferring graphene film on a target substrate introduced cracks and increased the corrosion | 21 |
| Ni | |||||||
| Monolayer and multilayer | ---- | CH4 | ---- | Cu | 0.1 M NaCl solution | Graphene protected metals differently | 20 |
| Ni | |||||||
| Monolayer | 950 | Hexane | 0.5 Torr | Cu | 0.1 M NaCl solution | Corrosion resistance of the graphene-coated Cu were 1.5 orders of magnitude higher than the uncoated Cu | 22 |
| Multilayer | 800 | CH4 | 10 Torr | Cu | Flow-boiling | Few-layer graphene acted as a protective layer even under vigorous flow boiling conditions. Oxidation was observed only along the grain boundaries of the graphene-coated substrate | 68 |
| Monolayer | 1000–1020 | CH4 | ---- | Cu | Oxidation at high temperatures | Graphene acted as a good oxidation barrier. Ineffectiveness of graphene coating over a long time of exposure to H2O and O2 | 23 |
| Multilayer | 940 | CH4 | 11 Torr | Cu | 3.5 wt.% NaCl solution | Good short term corrosion protection. Prolonging the immersion resulted in detaching the graphene film from the substrate | 16 |
| Few layer | 1000 | CH4 | 400 mTorr | Cu | 0.1 M Na2SO4 solution | Limited passivation provided by graphene due to its nanometre-size defects | 54 |
| Multilayer | 1000 | CH4 | 300 mTorr | Cu/Al | 0.1 M NaCl solution | Better protective properties of graphene coating on Cu compared to Al, due to the breakage of Al oxide film, causing the corrosion of Al to proceed rapidly | 52 |
| Monolayer | 700 | Ethylene | 5*10−7 mbar | Pt(100) | Air oxidation | Graphene layer stayed intact. Oxidizing agents diffused beneath the graphene layer | 41 |
| Water | |||||||
| NaCl solution | |||||||
| ---- | 1027 | Propane | ---- | Cu(111) | Atmospheric gases | Graphene acted as an attractive corrosion barrier in macroscale. Defective sites of graphene resulted in penetrating the atmospheric gases into the substrate | 24 |
| Multilayer | ---- | ---- | ---- | Ni | NaOH | Graphene coatings fail to protect the underlying metal in galvanic corrosion conditions | 25 |
| Multilayer | 940 | CH4 | 11 Torr | Cu | 3.5 wt.% NaCl solution | In a short-time exposure, graphene provided better protection for the polished Cu. Graphene on polished and unpolished Cu substrates failed to protect Cu in prolonged exposure | 26 |
| Multilayer | 850 | Acetylene | 10−1 mbar | Ni/SS | Boiling seawater | Superiority of the G/Ni/SS compared to Ni/SS in long term exposure. In the short/ medium-term, the performance of G/Ni/SS is comparable to Ni/SS. | 31 |
| Monolayer | 1000 | Propane (C3H8) | 100 mbar | Mono- and polycrystals Cu | Urban atmospheric conditions | No trace of copper oxide on the monocrystal Cu, while an oxide layer was developed on the polycrystal Cu due to the electrolyte intercalation at the graphene/Cu interface | 32 |
| Multilayer | 1000 | CH4 | Ambient pressure | Mo | 0.1 M NaCl solution | Graphene coating on the annealed Mo provided better protection against corrosion during the initial exposure times. After prolonged exposure, both graphene coatings on annealed and non-annealed Mo exhibited nearly the same corrosion inhibitive properties. | 92 |
| Multilayer | 600 | C2H2 | ---- | Cu | 3.5 wt.% NaCl solution | Corrosion current density of graphene-coated Cu fabricated at 400 °C is one order of magnitude lower than that of bare Cu | 72 |
| 400 | CH4 | ||||||
| Few-layer | 1000 | CH4 | Ambient pressure | Mo | 0.1 M NaCl solution | The lowest corrosion current density was calculated for Mo/Pt-G coating compared to Mo-G coating | 100 |
| Bi-layer | Pt-coated Mo | ||||||
| Bi-layer | 900 | Ethanol | 10 Torr | Cu | 3.5 wt.% NaCl solution | Corrosion rate of the coated Cu was 24 times smaller compared to the bare Cu | 71 |
| Cs-doped graphene | 1000 | CH4 | Ambient pressure | Cu | 0.1 M NaCl solution | Lower adhesion of Cs-doped graphene coating and decreased conductivity of graphene after doping with alkali metals that promoted the active dissolution of Cu. Reduced electron work function caused the Cu surface to become more electrochemically active. | 73 |
| N-doped graphene | 1050 | Cu(OAc)2 | ---- | Cu | Ambient environment | N-doped graphene on Cu substrate presented a long-term protection ability of the substrate in ambient condition. | 70 |
| Fluorinated graphene | ---- | ---- | ---- | Cu | 3.5 wt% NaCl solution | Prolonging the graphene protection ability of the substrate from corrosion | 60 |
Conclusions and Outlook
This review discussed the ability of CVD-grown graphene coatings to protect metal substrates during exposure to corrosive environments. Based on the various characteristics of graphene, i.e., high electrical and thermal conductivity, mechanical strength, chemical and thermal stability, optical properties, and impermeability, graphene was introduced as a candidate for opening a wide range of exciting applications. Although graphene has already been used in some real-life applications, applying it as a corrosive protection coating is one of the future works that faces obstacles for crossing the path from laboratory to commercialization.
Graphene coating on copper exhibited good protective properties and better corrosion stability than caesium-doped graphene coating (G/Cs) because the work function of G/Cs coating is lower, resulting from increasing the electron concentration of the graphene coating due to the Cs dopants and fewer layers, fewer defects, and larger domain size of graphene coating compared to the G/Cs coating.
Graphene coatings on both annealed and non-annealed molybdenum act as a corrosion barrier. The graphene coating on annealed Mo provided better protection against corrosion during the initial exposure than the graphene coating on non-annealed Mo. After prolonged exposure, graphene coatings on both annealed and non-annealed Mo exhibited nearly the same corrosion inhibitive properties.
Graphene coatings on both bare Mo and Pt-coated Mo exhibited good protective properties. The graphene coating on Pt-coated Mo exhibited higher values of polarization resistance and the lowest values of corrosion current density and corrosion rate because the graphene coating on Pt-coated molybdenum foil was bi-layer and had fewer defects. In contrast, the graphene coating on Mo foil was fewer layers with more defects, confirming the role of Pt thin film as a catalyst for CVD synthesis of high-quality protective graphene coatings.
Graphene coatings on aluminium exhibited poor protection, i.e., lower impedance values and higher corrosion current density than bare aluminium. This is a consequence of the weak adhesion of graphene coating and the galvanic corrosion of aluminium, which can occur when there is contact with a more noble metal or another electron conductor with a higher chemical potential than aluminium.
As a protective coating, one of the limitations of graphene is its failure after obtaining damages and defects. Therefore, in applying graphene as a protective coating, the coating should be high quality, without any defects, and it must be used in the circumstances that it does not face abrasion while protecting the surface. For producing a high-quality non-defective graphene layer in the CVD technique, various factors, i.e., growth temperature, growth pressure, precursors, number of graphene layers, type, morphology, and crystal structure of the substrate, etc., need to be taken into account. Furthermore, in the cases that graphene needs to be transferred to the target substrate after the growth, the transfer techniques must be monitored carefully to avoid any extra stress on the transferred graphene layer.
Another obstacle regarding using graphene as a corrosion barrier is the delamination of the protective coating from its substrates that facilitate the corrosive agents reaching the substrate. Future works must focus on finding approaches for enhancing the adhesion of the produced graphene to its substrate (whether smooth or rough), i.e., by functionalizing the substrate surface, doping graphene with some elements, changing the substrate surface morphology etc.
Moreover, almost all the reviewed articles presented here used graphene coating on a laboratory scale, which could be due to the limitations facing graphene mass production using the CVD technique. Therefore, one of the prospective future challenges will be finding a cost-effective, eco-friendly, and facile procedure for industrial-scale production of high-quality graphene. The roll-to-roll production of graphene has provided the chance for mass production of graphene that needs to be more developed for reaching the final goal of producing high-quality defect-free graphene on a large scale.
Another challenge that some researchers reported in applying graphene as a corrosion barrier was the failure of the coating in long-term exposure to the corrosive environment. In a short time, graphene acted as a reliable barrier, while by prolonging the exposure time, graphene failed to protect the substrate. Since long-term protection ability is required in all real applications, one of the future research directions should focus on addressing this issue.
Acknowledgments
The authors wish to thank all colleagues who contributed to the experimental results and analyses presented in this review. Their names can be seen in our mutual papers listed in the references. The authors also wish to acknowledge financial support by the Ministry of Education, Science, and Technological Development of the Republic of Serbia, Contract No. 451–03–9/2021–14/200135.