Abstract
Lithium ion batteries using liquid electrolytes often face challenges of safety issues. Polymer electrolytes can effectively solve this problem. Traditional preparation of polymer electrolytes is solution-casting method, which is complicated in practical application. Simultaneously, this ex-situ polymer electrolytes prepared by conventional method exhibits poor interfacial contact with electrodes. Fortunately, the emerging in-situ polymerization of solid state polymer electrolytes simplifies the preparation and forms an integrated interface for better interfacial compatibility in solid state lithium batteries. It is certain that solid state lithium batteries via in-situ polymerization exhibit various functionality: (1) forming integrated interface to enhance interfacial compatibility; (2) inhibiting the dissolution of transition metal ions; (3) suppressing the growth of lithium dendrites; (4) Improving the cycle performance of silicon anodes; (5) inhibiting the shuttling effect of polysulfides; (6) promoting battery performance of post-lithium batteries. Therefore, the review mainly considers and discuss the up-to-date research progress and insights on scientific issues underpinning solid state lithium batteries via in-situ polymerization strategy. Moreover, the challenges and perspectives of developing solid state lithium batteries via in-situ polymerization are discussed as well. We believe that this review will shed light on scientific and practical issues in the development of high-performance solid state lithium batteries.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Nowadays, lithium ion batteries have become one of the most promising energy storage devices due to their high operating voltage, high energy density, long cycle life, low self-discharge rate and no memory effect.1 However, state-of-the-art lithium ion batteries using liquid electrolytes often suffer from electrolyte leakage, volatilization, burning and even explosion owing to the intrinsic instability of liquid electrolytes. Consequently, it is now widely accepted that the replacement of conventional liquid electrolytes by solid electrolytes has become an ideal and effective method to promote safety performance of lithium batteries. Solid electrolytes mainly include inorganic solid electrolytes (ISEs) and solid polymer electrolytes (SPEs). ISEs mainly include oxides2 and sulfides3 Generally speaking, they often exhibited high ionic conductivity at room temperature, which almost reach the ionic conductivity level of liquid electrolytes. Despite their advantages, ISEs have not been widely used in high-energy lithium batteries due to their brittleness and extremely poor electrode/electrolyte contact.
Compared with ISEs, SPEs possess better flexibility and processability,4,5 which is beneficial to mass production for actual application. SPEs were composed of polymer matrix and lithium salts. Polymer segments with strong polar groups interact with lithium ions. Typically, lithium ions are transported through the amorphous portion of polymer matrix. Through repeated coordination-dissociation processes, lithium ions migrate directionally along with changes in conformation and free volume caused by segmental motion. Due to the plasticity of the polymer matrix, SPEs can also be made into a special shape as needed.6 All of these advantages make SPEs a more attractive application perspectives. However, the widely used polyethylene oxide (PEO)-based SPEs exhibited low ionic conductivity (10−8 S cm−1–10−6 S cm−1) at room temperature.7 Accordingly, there have been many efforts (blending, copolymerizing, cross-linking, branching, grafting and adding inorganic fillers to form organic-inorganic composite electrolytes) to improve room-temperature ionic conductivity of PEO-based SPEs, but still cannot meet the practical requirements of high-performance lithium batteries. In recent years, researchers pay more attention to develop advanced SPEs (such as polysiloxane, aliphatic polycarbonate, poly (vinylidene fluoride), succinonitrile and etc.) to further improve room-temperature ionic conductivity and other electrochemical performance.8–13 In the 1970s, Feuillade et al. first proposed adding a plasticizer to SPEs to prepare gel polymer electrolytes (GPEs), which not only significantly improved room-temperature ionic conductivity, but also possess superior safety issues. At present, polyethylene oxide (PEO), polymethyl methacrylate (PMMA), polyvinylidene fluoride (PVDF), polyacrylonitrile (PAN) and related co-block polymers are widely used as polymer matrix for preparing GPEs.14–16
With respect to SPEs, the widely used preparation technique is solution-casting method. The detailed preparation process is as follow: Firstly, a certain amount of polymer matrix and lithium salts were dissolved in organic solvent to obtain homogeneous solution under stirring. Subsequently, the obtained homogeneous solution was poured into glass plate or Teflon plate and then dried at an elevated temperature (i.e. 80 °C) to remove liquid organic solvent. Last, SPEs were obtained after peeling off them from glass plate or Teflon plate. From the above we can clearly see that solution-casting method is complicated. Simultaneous, this process usually accompanied by the volatilization of organic solvents, which is detrimental to the environment. In addition, the poor electrolyte/electrode interfacial contact in solid state lithium batteries using this method is a common issue, mainly originating from the ex-situ assembly technology of solid state lithium batteries. These three factors greatly hampered large-scale preparation of solid state lithium batteries. Reducing the impedance of electrode/electrolyte interface and maintaining interfacial compatibility is still an urgent problem to be solved for high-performance solid state lithium batteries. Similarly, traditional preparation strategies of GPEs mainly include casting method, phase inversion method, electrospinning method and etc. After that, plasticizer (i.e. liquid electrolyte) is introduced to make GPEs. These methods are also complicated in operation and harsh in conditions, making it difficult to implement in actual production. More importantly, all of the above-mentioned methods are ex-situ preparation methods, resulting in complex preparation process, severe environmental pollution and poor electrode/electrolyte interface contact.
Accordingly, in-situ polymerization of polymer electrolytes effectively solves the above-mentioned problems. In the process of in-situ polymerization, monomers, plasticizer (or not), lithium salts and initiators are combined into a precursor solution, and then the precursor solution is injected into the lithium batteries. After the precursor solution fully wets the electrodes, the precursor polymerized in situ under certain external conditions to obtain gel/solid polymer lithium batteries. In-situ generated polymer electrolytes by the polymerization of small-molecule liquid precursors into macromolecular polymers can effectively reduce the fluidity of the electrolytes, thereby improving the safety of the batteries. This technology not only simplifies the preparation process, but also maintain interfacial compatibility and stability of solid state lithium batteries. In terms of polymerization mechanism, in-situ polymerization includes free radical polymerization, cationic polymerization, anionic polymerization, gelation-factor polymerization, thermochemical cross-linking polymerization without initiator, gamma-ray initiated polymerization without initiator and electrochemical initiated polymerization without initiator.17–46 In-situ polymerization of polymer electrolytes have been used in a variety of solid state lithium batteries. In the most recent years, a significant progress has been made for in-situ polymerization towards solid state lithium batteries, lithium metal batteries and high-voltage lithium batteries. It is certain that solid state lithium batteries via in-situ polymerization exhibits various functionality: (1) forming integrated interface to enhance interfacial compatibility17–25; (2) inhibiting the dissolution of transition metal ions26–28; (3) suppressing the growth of lithium dendrites29–35,43; (4) improving the cycle performance of silicon anodes36,37; (5) inhibiting the shuttling effect of polysulfides38–43; (6) promoting battery performance of post-lithium batteries.44–47
In the following sections, we focus on current research progress and scientific issues of in-situ polymerization for integration interface and interfacial protection towards solid state lithium batteries. Finally, the current challenges and future perspectives of solid state lithium batteries via in-situ polymerization strategy will also be prospected.
Forming Integrated Interface to Enhance Interfacial Compatibility
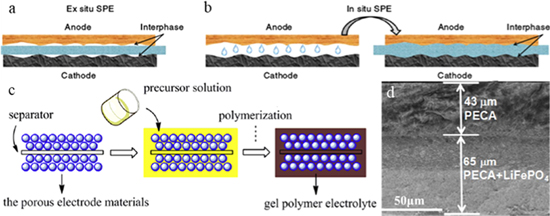
Solid state lithium batteries have received extensive attention owing to their superior safety and relatively high energy density. However, there is no sufficient wettability between electrolyte/electrode interfaces when using ex-situ assembly method. Poor interfacial contact generated during the ex-situ assembly process. The reason is that there are many gaps between the rigid interface of the ex-situ SPEs and the electrodes (Fig. 1a). As a result, the poor solid-solid contact limited the electrochemical performance of solid state lithium batteries. Fortunately, in-situ polymerization can greatly reduce the solid/solid interface impedance and meet the requirements of solid state lithium batteries. The precursor solution is injected into the battery, and the precursor solution sufficiently wets the electrodes before the polymerization (Figs. 1b and 1c).
Figure 1 (a) Ex-situ SPE and (b) in-situ SPE contact with the electrodes 31 (c) Schematic diagram of in-situ polymerization of GPE.17 (d) Typical SEM images of the cross-section of PECA electrolyte/LiFePO4 cathode.19
Download figure:
Standard image High-resolution imageIn 2017, Prof. Cui et al. demonstrated poly(ethylene glycol) diglycidyl ether/LiODFB solid state polymer electrolyte via a facile cationic polymerization initiated by LiODFB.19 It was found that LiFePO4/Li metal battery using this SPE delivered superior cycling stability (capacity retention of 74.2% after 100 cycles). Moreover, the interface resistance between this SPE and the lithium metal anode remains stable even after 20 d during the long-term cycles. In addition, SEM images suggest LiFePO4 cathode and Li anode maintain close contact with solid polymer electrolyte even after 100 cycles, which is beneficial to maintain interfacial integration and reduce the interface impedance. Similarly, this group developed polyethyl cyanoacrylate based polymer electrolyte (PECA-GPE) by in-situ anionic polymerization initiated by lithium power.18 Ionic conductivity of this in-situ polymerized PECA-GPE was 2.7 × 10−3 S cm−1 at room temperature. More importantly, low-viscosity ECA-based precursors can easily penetrate into LiFePO4 cathode and then provide continuous ion transport channels. Moreover, the cross-section of SEM images showed good interfacial contact between LiFePO4 electrode and PECA electrolyte (Fig. 1d). Therefore, PECA-GPE formed by in-situ polymerization have continuous contact with the active material of the electrode and form continuous ion transport channels, which greatly reduces the interfacial impedance of the electrode/electrolyte interface. As a result, LiFePO4/Li metal battery employing this PECA-GPE exhibited superior cycling stability (capacity of 90% after 100 cycles). Whether this in-situ polymerization of PECA-GPE can be used in high-voltage cathodes (i.e. 4.4 V LiCoO2)/Li metal batteries is worth further study.
It is certain that vinylene carbonate (VC) is a promising additive of liquid electrolytes to stabilize the anodes (such as graphite, Li metal and silicon). Considering the positive effect of VC on interfacial compatibility, In 2017, Prof. Cui et al. proposed a simple in-situ polymerization method to prepare a PVCA-based SPE.20 After 150 cycles, the capacity retention of LiCoO2/Li battery with this SPE was 84.2%. Although PVCA-SPE has good interfacial compatibility with 4.3 V LiCoO2 cathode and Li metal, the rate capability of PVCA-SPE-based SSBs at room temperature are not ideal. The room temperature ionic conductivity of PVCA-SPE is only 2.23 × 10−5 s cm−1. Therefore, they proposed a polyethylene carbonate based GPE to increase room-temperature conductivity (5.59 × 10−4 s cm−1).21 Moreover, the interface impedance between in-situ PVCA-based SPE and ex-situ PVCA (solution casting process) based GPE and lithium metal was compared. The results showed that the in-situ PVCA-based GPE and lithium metal resistance increased slightly (from 190 Ω to 320 Ω) after 30 d of in-situ polymerization. In contrast, the interfacial impedance of the ex-situ PVCA-based GPE increased significantly after 30 d (from 1400 Ω to 5800 Ω). This advantage of in-situ polymerization was more clearly demonstrated.
Zhou et al. prepared cross-linked trihydroxypropane trimethacrylate (TMPTMA)-based GPE by in-situ thermal polymerization.22 LiCoO2/graphite full battery using this GPE has a capacity retention rate of 83% and a discharge capacity of about 107 mAh g−1 after 100 cycles at 0.2 C. More importantly, Young-Soo Kim et al. found that cyanoethyl polyvinyl alcohol (PVA-CN) can be irreversibly gelled without the addition of any additional initiators at 60 °C.23 Also, the PVA-CN-based GPE has an ionic conductivity of 8.63 × 10−3 S cm−1, which is almost equivalent to that of liquid electrolytes at room temperature. However, the gelation mechanism of PVA-CN was still unknown.
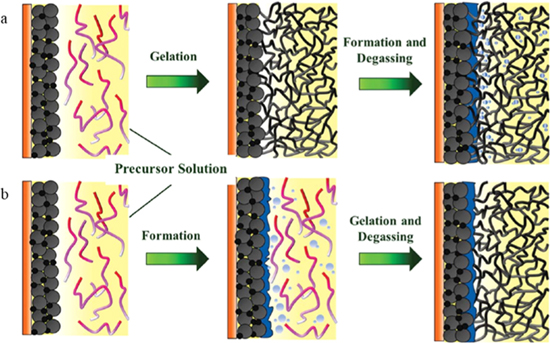
To unveil this gelation mechanism of PVA-CN, Zhou et al. proposed that the PVA-CN based organogel is formed by in-situ cationic polymerization of cyano resin initiated by PF5 (decomposed product of LiPF6).24 In addition, Zhou et al. further optimized the electrode/GPE interface. They compared the interfacial impedances of the gelation process before and after the formation of the cells, and found that gelation after the formation of the cells is more conducive to the formation of a stable interface. Gelation after battery formation inhibits the formation of SEI with high impedance and reduces bubbles during the reaction trapped in the GPE (Fig. 2). The group also designed a PVA-CN based hierarchical SPE based on nitrile material (SEN).25 The in-situ polymerized PVA-CN is uniformly dispersed in the SN-based SPE to form a skeleton, which greatly enhances the mechanical properties of the SN-based SPE and maintains its quasi-solid state under high temperature conditions. LiFePO4/Li battery with SEN has a discharge capacity of 149.6 mAh g−1 at 0.1 C, and the capacity retention is 96.7% after 100 cycles. In contrast, the liquid electrolyte (1 M LiPF6-EC/EMC/DMC)-based LiFePO4/Li battery delivers initial discharge capacity of 160.8 mAh g−1 (higher than SEN's 154.6 mAh g−1), but the capacity retention was only 93.2%, indicating good contact of SEN/electrode interface.
Figure 2. Schematic diagram of graphite electrode/GPE interface when gelation: (a) before and (b) after battery formation.24
Download figure:
Standard image High-resolution imageOverall, in-situ polymerization of polymer electrolytes can form integrated structure and then enhance interfacial compatibility of lithium batteries. It should be noted that all the results were obtained in coin cells. Whether the same effect can be achieved in soft-pack lithium batteries needs to be investigated comprehensively. With respect to integration, the volume change of polymer electrolyte is also worthy of attention before and after in-situ polymerization.
Inhibiting the Dissolution of Transition Metal Ions
In-situ polymer electrolytes can effectively inhibit the dissolution of transition metal ions from cathodes during long-term cycles. First, in-situ polymer electrolytes can penetrate deeply into the pores of the electrode materials, which can physically maintain the morphological appearance of the positive electrode. In addition, the non-aqueous plasticizer is encapsulated in the polymer network to avoid continuous side reactions with cathodes. At present, the positive electrode of lithium battery is mainly composed of transition metal oxides containing Li. In the process of Li+ extraction and embedding, the transition metal element changes the valence state to maintain the electrical neutrality of the positive electrode material. Ni, Co, and Mn are the three most common transition elements in lithium-ion batteries, such as the valence state change process of Mn:  But the dissolution of transition metals is a very common phenomenon during the cycling of lithium-ion batteries. The dissolved transition metal element migrates to the surface of the negative electrode, destroying the SEI film, causing irreversible attenuation of the capacity. Due to the John-Teller effect of Mn, more research has focused on the dissolution of Mn. In general, the solution to the above problem is to coat a surface of the positive electrode with a polymer layer to inhibit the dissolution of Mn by both physical and chemical aspects.6 The in-situ PEs complete the surface coating process of the positive electrode during the in-situ polymerization process, which simplifies the battery assembly process while achieving the same or better effect.
But the dissolution of transition metals is a very common phenomenon during the cycling of lithium-ion batteries. The dissolved transition metal element migrates to the surface of the negative electrode, destroying the SEI film, causing irreversible attenuation of the capacity. Due to the John-Teller effect of Mn, more research has focused on the dissolution of Mn. In general, the solution to the above problem is to coat a surface of the positive electrode with a polymer layer to inhibit the dissolution of Mn by both physical and chemical aspects.6 The in-situ PEs complete the surface coating process of the positive electrode during the in-situ polymerization process, which simplifies the battery assembly process while achieving the same or better effect.
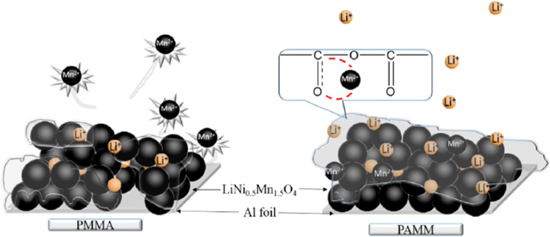
In 2017, Prof. Cui et al. proposed a strategy to solve the challenges of LiNi0.5Mn1.5O4 battery system by in-situ generated cross-linking poly (acrylic anhydride-2-methyl-acrylic acid-2-oxirane-ethyl ester-methyl methacrylate) (PAMM) electrolyte.26 Electrochemical properties LiNi0.5Mn1.5O4/cross-Linking PAMM/Li metal batteries were investigated comprehensively. TEM image of cycled LiNi0.5Mn1.5O4 electrode indicated that a uniform and robust cathode electrolyte interface (CEI) formed on the surface of cathode (Fig. 3). The reason is that anhydride and acrylate groups containing polymer system polymerizes on the surface of the cathode, and a stable cathode/electrolyte interface is formed due to the O=C–O–M (M=Ni, Mn) bonds (Fig. 3). Moreover, the first reversible discharge capacity of the battery was 131.7 mAh g−1. And the capacity retention after 500 cycles reached 78.9% at potential range of 3.5 V−5 V. In contrast, the reversible discharge capacity of the LiNi0.5Mn1.5O4/PMMA/Li battery was 123.1 mAh g−1, and the capacity retention was only 63.44% after 500 cycles. These results demonstrate that PAMM forms a stable passivation layer on the surface of the cathode, which inhibits the decomposition of the electrolyte and the dissolution of Mn2+.
Figure 3. Illustrations of working mechanism of different electrolytes.26
Download figure:
Standard image High-resolution imageSubsequently, Prof. Cui's group demonstrated poly(vinyl carbonate)-Li10SnP2S12 composite solid state electrolyte (PVCA-LSnPS) by in-situ polymerization.27 LiFe0.15Mn0.85PO4 (LFMP)/Li metal cells using this PVCA-LSnPS and liquid electrolyte (1 M LiPF6 in EC:DMC:DEC of volume ratio1:1:1 ) as electrolytes were installed and tested comprehensively. The results show that the battery using PVCA-LSnPS is more stable than liquid electrolyte. Furthermore, Element mapping analysis of the cycled PVCA-LSnPS composite electrolyte showed that Mn element was not present in the composite electrolyte. These results prove that the in-situ generated PVCA-LSnPS can effectively inhibit the dissolution of Mn when using the LFMP cathode.
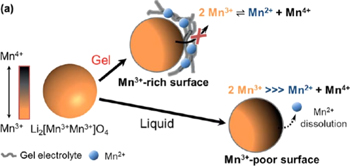
The main way to inhibit Mn2+ dissolution above is to chelate metal ions and inhibit electrolyte attacks. In order to fundamentally inhibit the dissolution of Mn2+, Prof. Cho et al. presented a metal-ion-chelating organogel electrolyte, thermally gelated within cells.28 The organogel electrolyte was functionalizing cyanoethyl polyvinyl alcohol (PVA-CN) with 2-pyrrolidone-5-carboxylic acid (PCA) as a chelating moiety (Pyrd-PVA-CN) characterized by in-situ gelation in LiPF6-based carbonate electrolytes. As we all know, the main reasons for the dissolution of Mn2+ from the cathode material is the disproportionation reaction of Mn3+. Therefore, the author solves the above problems from two aspects as in Fig. 4: (1) capturing metal ions dissolved from the cathode and deposited on the anode due to strong interaction between pyrrolidone groups and metal ions; and (2) suppressing metal dissolution from the cathode due to Le Chatelier's disproportionation depression. The strong ion-dipole interaction of Mn2+ with Pyrd supports the chelating ability of Pyrd-PVA-CN. Moreover, the thermodynamic properties of Mn2+-pyrd complex are better than the solvation of Mn2+, which effectively inhibits the dissolution of Mn2+. In the presence of this metal-ion-chelating gel, the capacity retention of the Li2Mn2O4 battery has been significantly improved. Therefore, it can be confirmed that the in-situ generated functional gel electrolyte can effectively stabilize the transition metal-based cathode materials.
Figure 4. The process of gel electrolyte inhibiting Mn2+ dissolution.28
Download figure:
Standard image High-resolution imageSuppressing the Growth of Lithium Dendrites
It is well known that SEI play an important role in the electrochemical performance of lithium batteries. The ideal SEI should possess the following functions: (1) uniform morphology and composition distribution, and high ionic conductivity; (2) good contact with the anode and elasticity to adapt to volume changes of the active material. The rupture of the unstable SEI leads to the continuous growth of lithium dendrites, which will accelerate the attenuation of the battery capacity. The most common problems in the interface between electrodes and solid electrolyte are poor interfacial stability, low ionic conductivity and growth of lithium dendrites. In-situ generated polymer electrolytes can form a close contact interface with the varied electrodes and induce the formation of a stable SEI to inhibit the growth of lithium dendrites.
During the in-situ polymerization process, the composition of the SEI film can also be adjusted to make the resulting SEI film more stable and possess high ionic conductivity. Prof. Lee et al. assembled LIBs with GPEs cured by in-situ chemical crosslinking using a mixture of polyethy-leneimine (PEI) with amine groups and poly (ethylene glycol) diglycidyl ether (PEGDE) with epoxy groups in fluoroethylene carbonate-containing liquid electrolyte.29 They investigated the surface of the carbon anodes cycled in liquid electrolyte and gel electrolyte by XPS measurements. The results show that the fluorinated carbonate in the cross-linked gel polymer electrolyte can reduce the formation of LiF with poor conductivity and increase the formation of lithium carbonate with high conductivity. Moreover, Prof. Cui et al. prepared a solid state polymer electrolyte based on poly (tetrahydrofuran) (PTHF)-LiClO4 (PTSPE) via an in-situ polymerization strategy using BF3 as an initiator.30 Galvanostatic lithium plating/stripping examination of in-situ prepared PTSPE based cell exhibits a stable over potential of 7 mV over 600 h at the current density of 0.05 mA cm−2. In addition, a smooth surface without lithium dendrite is observed on the surface of lithium metal anode. In contrast, the over potential of the ex-situ prepared PTSPE based cell fails due to a short circuit after the 70 h. These results fully suggest that the in-situ formed solid state polymer electrolyte can protect lithium metal anode. More importantly the XPS measurements results demonstrate that a relatively large fraction of LiF and B-O species derived from BF3 decomposition were formed on the surface of the cycled Li metal using in-situ formed PTSPE. These species are beneficial to form effective SEI film on lithium metal. Thus, LiF is a controversial species for the formation of stable SEI. It can be concluded that an appropriate amount of LiF is beneficial to the stability of the SEI film, but an excessive amount of LiF causes a large interface impedance. Zhao et al. reported that in-situ ring-opening polymerization of molecular ethers initiated by cationic aluminum species to produce SPE.31 It was found that this SPE can form a more stable SEI film than the original liquid electrolyte. The SEI film consists of LiF, aluminum complex (AlF3 and Al2O3) that can stabilize the lithium electrode, and very small Li2S with poor ionic conductivity.
All of the above-mentioned results demonstrate that in-situ polymerization of polymer electrolytes can regulate the composition of SEI, making it more conducive to the interfacial compatibility of the negative electrodes and reduce the interfacial impedance.
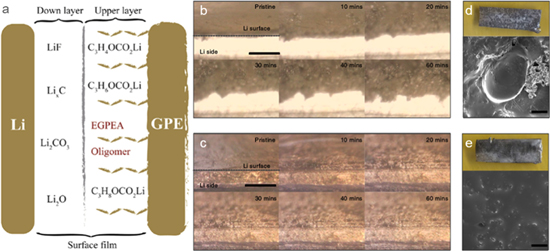
In addition to adjusting the composition of SEI, in-situ generated polymer electrolytes can also physically enhance the stability of the SEI. The integrated interface formed by in-situ polymerization is in close contact with lithium metal anode. The polymer network with strong mechanical properties can effectively suppress the volume change of lithium metal anode and then blocks the corrosion of lithium metal anode by the electrolyte or other intermediate products. Furthermore, when the SEI film is formed during the initial charge-discharge cycle, a polymer-enhanced SEI formed. Therefore, lithium dendrites are effectively suppressed because of the continuous inhibition of polymer-enhanced SEI. Prof. Niu et al. fabricated poly (ethylene glycol phenyl ether acrylate) (PEGPEA) based GPEs via in-situ polymerization. EGPEA is uniformly dispersed in the precursor solution prior to in-situ polymerization.32 Thus, after the formation of GPEs, the solvent molecules, the oligomer of EGPEA and the PEGPEA fragment are tightly bound to the surface of the lithium metal. The PEGPEA-based polymer network effectively enhances the mechanical strength of SEI and thus suppresses the growth of lithium dendrites (Fig. 5a).
Figure 5. (a) Schematic diagram of polymer-enhanced SEI film32 and Li electrodeposit morphology in (b) and (d) liquid electrolyte and in (c) and (e) poly-DOL SPE.31
Download figure:
Standard image High-resolution imageKong et al. proposed a novel in-situ fabrication concept, which is based on the electro-polymerization of a pristine liquid organic electrolyte of 1 M LiTFSI /DOL/DME (2:1 by weight).33 FTIR results and SEM characterization confirmed the formation of a smooth, small amounts of cracks and a uniform protective polymer surface layer on the lithium metal anode. Subsequently, many studies have focused on upgrading traditional DOL/DME electrolytes to obtain pol-DOL-based PEs. Liu et al. proposed a method for upgrading conventional electrolyte (DOL/DME LE) to GPE by in-situ ring-opening polymerization between LiPF6 and DOL in the presence of trace water.43 This GPE exhibits superior interfacial compatibility with Li metal anode. It is successfully applied into a series of LMBs with the cathodes of sulfur, LiFePO4, and LiNi0.6Co0.2M0.2O2 (NCM622), exhibiting universality and encouraging commercialization prospects. Zhao et al.12 upgraded traditional LiTFSI/DOL LE to SPE by in-situ cationic ring-opening polymerization of molecular ethers initiated by aluminum compounds without water participation.31 The morphology evolution of the Li metal anode after electrodeposition observed by real-time optical microscopy and SEM is vividly shown in Figs. 5d and 5e). In the Li/Li symmetric battery (Figs. 5b and 5c), the Li anode using LE exhibits moss-like lithium electrodeposition only after ten minutes, and then the lithium dendrites gradually grow visible in the naked eye. In contrast, the Li metal anode using poly-DOL SPE was flat. It was demonstrated that in-situ formed PEs can indeed improve the uniform deposition of Li by the inherent elasticity of the polymer.
In addition, crosslinked polymers formed via in-situ polymerization strategy have also been proved to be an effective solution to promote uniform deposition of lithium. Fan et al. also designed a dual-salt (LiTFSI-LiPF6) GPE with cross-linked polymer network copolymerized by PEGDA and ETPTA.34 A high ionic conductivity (5.6 × 10−4 s cm−1 at room temperature) is obtained simultaneously with a robust and conductive SEI on the lithium metal surface, which achieves 87.93% capacity retention after cycling for 300 cycles of battery and uniform lithium deposition. The above studies have proved that cross-linked polymers are an effective research direction to improve the interfacial compatibility of GPE and Li anodes.
Furthermore, single-ion-conducing polymer electrolytes can also effectively suppress the growth of lithium dendrites. Zhou et al. reported a hierarchical multifunctional polymer electrolyte prepared by in-situ polymerization.35 The electrolyte is composed of in-situ prepared (1-[3-(methacryloyloxy) propylsulfonyl]-1-(trifluoromethanesulfonyl)imide (LiMTFSI)-pentaerythritol tetraacrylate (PETEA))-based GPE in poly(3,3-dimethylacrylic acid lithium) (PDAALi)-coated glass fiber membrane. Its lithium ion transference number is as high as 0.75, which can almost be called a single-ion-conductor. On the one hand, the cross-linked polymer network formed in situ generates a uniform lithium ion flux and suppresses volume changes caused by lithium ion de-intercalation. On the other hand, high lithium ion transference number can also reduce the concentration polarization caused by anion migration. The Li/Li symmetrical battery assembled with this GPE maintains a low and stable voltage hysteresis at a current density of 0.5 mA cm−2 for 500 h without significant oscillation. The author also studied the morphology of lithium deposits on Cu substrates with a plating capacity of 5 mAh cm−2 by SEM. It was observed that the Li deposits in Li/Cu cell using this GPE exhibited the morphology of densely clustered nodule-like particles (∼7 μm) without dendrite formation.
In summary, the integrated interface of in-situ polymerized polymer electrolytes and anodes can avoid dendrite growth caused by local large currents, and can regulate the formation of stable SEI membranes during in-situ polymerization. The elastic polymer network closely attached to the anode cooperates with SEI to better accommodate the volume change of the anodes during charge/discharge process. In addition, PEs with crosslinked polymer networks can also enhance the interfacial compatibility of electrolytes with electrodes through the synergy of two polymer networks. At the same time, single-ion-conducting polymer electrolytes with high lithium ion transference number can also suppress the growth of lithium dendrites by reducing concentration polarization. All of the above advantages indicate that the in-situ generated polymer electrolytes can effectively improve the anode/electrolyte interface performance and then stabilize the anode surface, thereby improving the electrochemical performance and safety issues of solid state lithium metal batteries.
Improving the Cycle Performance of Silicon Anodes
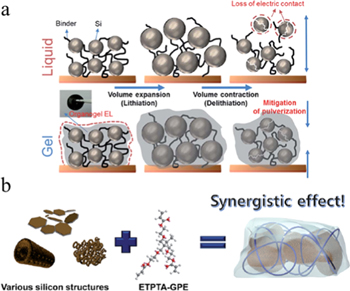
Silicon is a low-cost and abundant anode material with extremely high theoretical capacity (3579 mAh g−1 based on Li15Si4 at room temperature), low lithiation voltage (∼0.3 V). However, the huge volume expansion (about 400%) of silicon anodes during electrochemical lithiation tends to cause pulverization and cracking of silicon particles, which hinders the application of silicon anodes. In-situ polymer electrolytes can improve the cycling stability of lithium ion batteries with silicon as the anode. In 2016, Prof. Cho et al. developed PVA-CN-based GPE to a silicon-based battery using high-loading silicon.36 Since the PVA-CN based GPE provides an additional cohesive force between the silicon powder and the carbon black (Fig. 6), even after the specific gravity of the binder and the conductive agent is lowered, no crack of the negative electrode is observed even after 50 cycles. In contrast, silicon anodes in conventional liquid batteries suffer from severe cracks. Therefore, this PVA-CN based GPE effectively improved the capacity retention of silicon-based batteries.
Figure 6. (a) Schematic diagram of morphological changes of silicon electrodes in lithiation and desulfurization processes in liquid and gel electrolytes.36 (b) Schematic diagram of the synergistic coupling of various silicon anode materials (mesoporous, microporous, 2d plates) and crosslinked ETPTA polymer mediated GPEs.37
Download figure:
Standard image High-resolution imageIn addition to polymer electrolytes, nanostructured silicon is also an effective method to solve the challenges of silicon anode materials. In 2016, Prof. Taesoo et al. applied nanostructured silicon together with in-situ generated GPE in a silicon-based battery (Fig. 6).37 They added a certain amount of ethoxylated trimethyl propyl triacrylate (ETPTA) monomer to the liquid electrolyte, and then injected the precursor solution into the battery to thermally crosslink in situ to form ETPTA-based GPE. It was found that the combination of silicon/GPE delivers much better cycle performance than that of silicon/liquid electrolyte. The discharge specific capacity of the silicon/GPE still exceeded 2000 mAh g−1 after 100 cycles, and the volume expansion of the silicon anode is also significantly controlled.
Inhibiting the Shuttling Effect of Polysulfides
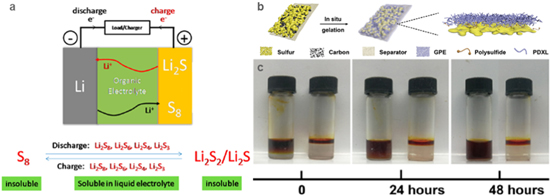
Lithium-sulfur batteries are rechargeable batteries based on a multi-electron reaction in which sulfur is converted to lithium sulfide. Figure 7a shows a series of polysulfides (such as Li2Sx, 2 < x < 8) are generated by complex disproportionation and exchange reactions during the charge and discharge.38 The soluble long-chain polysulfides Li2Sn (n = 4–8) pass through the electrolyte to the surface of lithium metal anode under the action of a concentration gradient, and then reduced to the short-chain polysulfides Li2Sx (x = 2–4). Under the action of the electric field and concentration difference, the short-chain Li2Sx will again return to the sulfur electrode through the electrolyte, and will be oxidized again to the long-chain polysulfide Li2Sn at the sulfur electrode. This phenomenon is called "shuttle effect of polysulfides".
Figure 7. (a) Charge and discharge diagrams of rechargeable lithium-sulfur batteries, and the production of soluble polysulfides during charging and discharging.38 (b) In-situ polymerization schematic diagram of battery.43 (c) Permeation behavior of Li2S8 in LE (left) and GPE (right).43
Download figure:
Standard image High-resolution imageOn the one hand, the shuttling effect of polysulfides consumes the sulfur active material, hinders electron and ion transport. On the other hand, the polysulfides diffuse to the lithium electrode to react with the metallic lithium and destroy the SEI film, causing severe self-discharge of lithium-sulfur batteries. The two most common methods used to address the polysulfide shuttling effect in previous studies include39: (1) Combine the porous carbon material with sulfur to improve the adsorption and confinement of polysulfides; (2) Form a barrier layer between the separator and the sulfur electrode, to hinder the diffusion of polysulfides to the lithium electrode. Although the above-mentioned methods can effectively improve the cycling stability of lithium-sulfur battery, the introduction of additional components significantly reduces the content of sulfur in the system, lowing the energy density of the battery. Fortunately, in-situ polymer electrolytes propose a new idea for suppressing the shuttling effect of polysulfides for developing high-performance lithium-sulfur batteries.
Compared with common liquid electrolytes, solid electrolytes can effectively suppress the dissolution of polysulfides. The permeation behavior of Li2S8 in DOL/DME LE and poly-DOL-based GPE is shown in Fig. 7c.43 For example, a polymer network of PEs formed in situ penetrates into the sulfur cathode to form a closed cathode chamber that blocks the polysulfides at the interface between the cathode and the electrolyte (Fig. 7b).43 Furthermore, in-situ formed polymer electrolytess can chemically interacts with polysulfides, effectively suppressing the shuttling effect of polysulfides.
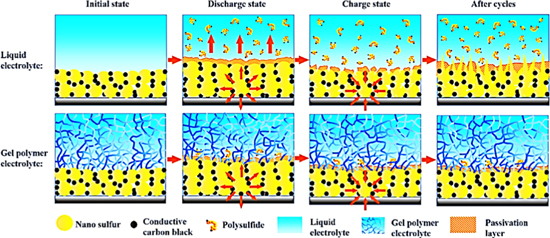
In 2016, Liu et al. reported novel pentaerythritol tetraacrylate (PETEA)-based GPE via in-situ synthetic strategy.40 Ionic conductivity of this GPE is 1.13 × 10−2 s cm−1 at room temperature. This GPE forms a compact structure with the electrodes and then effectively inhibits the shuttling effect of soluble polysulfides. These improvements give the polymer Li-S battery a low electrode/GPE interface resistance, high rate capacity (601.2 mAh g−1 at 1 C) and high capacity retention (81.9% after 400 cycles at 0.5 C). As can be seen from Fig. 8, for the Li-S battery using LE, the breakdown-reconstruction of the passivation layer and the continuous growth of dendrites on the surface of the sulfur electrode, accelerate the dissolution of polysulfide and increase the interface impedance. In contrast, in a Li-S battery using PETEA-based GPE, a high mechanical strength PETEA-based gel matrix is pre-coated on the surface of the cathode, which greatly enhances the flexibility of the passivation layer to butterfly the volume change of the sulfur particles. Therefore, PETEA-based GPE combined with this flexible passivation layer can effectively inhibit continuous interfacial reaction and polysulfides dissolution. More importantly, the strong interaction between lithium sulfide and the oxygen donor atoms in ester (C=O) groups of polymerized PETEA also makes a great contribution to the immobilization of polysulfides. In order to further mechanical strength of GPE to eliminate commercial separators and to continuously optimize ion channels in porous media to prevent polysulfide diffusion, this group presented an acrylate-based hierarchical electrolyte (AHE) for Li-S batteries.41 The AHE was assembled by integrating a PPETEA GPE with a polymethyl methacrylate (PMMA)-based electrospun fiber network. It dramatically enhances the overall electrochemical performance of Li-S batteries (91.9% after 500 cycles at 3 C).
Figure 8. The immobilization mechanism for polysulfides by capitalizing on PETEA-based GPE as electrolyte.40
Download figure:
Standard image High-resolution imageBesides, other studies about upgrading conventional liquid electrolytes were also conducted accordingly. In 2017, Prof. Li et al. demonstrated high-performance lithium-sulfur (Li-S) batteries by using acidized carbon nanotube paper (ACNTP) to induce in-situ polymerization of ether-based DOL/DME liquid to grow an ion-selective solid barrier, to seal in soluble polysulfides on the cathode side.42 The capacity decay rate of the Li-S battery with the in-situ barrier at a high current density of 1675 mA g−1 was 0.1% per cycle and a high Coulombic efficiency of 99% was achieved. In 2018, Prof. Guo et al. proposed a new strategy to upgrade traditional liquid electrolytes to GPE by in-situ polymerization.43 This new strategy is converting traditional ether-based 1,3-dioxolane (DOL) and 1, 2-dimethoxyethane (DME) LEs into a novel quasi-solid GPE simply with the addition of commercial lithium hexafluorophosphate (LiPF6). The study demonstrates that a polymer backbone formed in situ can encapsulate polysulfide in the cathode chamber. As a result, after 500 cycles at 0.5 C, the discharge specific capacity of lithium-sulfur battery using this GPE remains 741 mAh g−1 with an excellent capacity retention of 73.7%.
In general, in-situ generated polymer electrolytes can effectively inhibit the shuttling effect of polysulfides and improve cycling stability of lithium batteries. It is also to be expected whether it can be used in sodium sulfur batteries.
Promoting Battery Performance of Post-Lithium Batteries
Although lithium batteries are currently the most competitive secondary batteries, they still need to be further improved especially in terms of safety, cost, environmental friendliness and energy density. Post-lithium batteries including lithium sulfur batteries, lithium air batteries, sodium ion batteries, magnesium ion batteries, zinc ion batteries and etc., have become an inevitable trend. Apart from rechargeable lithium batteries, in-situ generated polymer electrolytes can also be used in other post-lithium batteries, such as sodium batteries, lithium air batteries, and magnesium batteries. In 2017, Prof. Cui et al. demonstrated a novel polysulfonamide-supported poly(ethylene glycol) divinyl ether polymer electrolyte by in-situ cationic polymerization strategy.44 The monolithic Na3V2(PO4)3/MoS2 full battery delivered superior cycle performance (capacity retention of 84% after 1000 cycles) at 0.5 C. In 2018, Prof. Zheng et al. prepared a novel flame retardant gel polymer electrolyte (FR-GPE) by in-situ crosslinking of a phosphorus-based crosslinking agent and alkyl acrylate monomer.45 It was found that the Na3V2(PO4)3/Na metal battery using this FR-GPE exhibited a high capacity retention (69.2% for a long period of 4500 cycles). Above-mentioned results fully indicate that in-situ polymerization of polymer electrolytes can endow excellent electrochemical properties in sodium batteries, especially in terms of improving long-term cycling stability.
Lithium air batteries (LABs) have the advantages of ultra-high energy density, environmental friendliness and low cost, but there are still many problems to be resolved. Since LABs are an open system, problems such as evaporation and oxidation of the electrolyte, moisture in the air, reaction of CO2 with lithium metal should be given extra attention. In-situ formed polymer electrolytes enable LABs a long cycle life in ambient air and flexibility. In 2018, Prof. Wang et al. presented a LAB with a tetraethylene glycol dimethyl ether (G4) based gel polymer electrolyte, in which the gel is formed through cross-linking reaction between liquid TEGDME and lithium ethylenediamine (LiEDA).46 It was demonstrated that in-situ formed gel polymer electrolyte guarantees excellent interfacial contact between electrodes and electrolyte. Furthermore, the G4 gel polymer electrolyte can also effectively prohibit the penetrating of moisture and CO2 due to the dense surface and CO2 capture group (-NH2). As a result, the diffusion rates of moisture and CO2 from the air to Li metal anode was obviously slowed down, and thus improving the cycle life of LABs in ambient air. The LABs with G4 gel polymer electrolyte deliver a stable cycle performance (more than 1175 h) in ambient air. In addition to improving the electrochemical performance of LABs, this integrated polymer electrolytes can also be adapted to the needs of wearable devices due to their inherent plasticity and flexibility in future.
Similarly, in-situ polymerization of polymer electrolytes can also be used in magnesium batteries to promote electrochemical performance. In 2019, Prof. Cui et al. conjugated polytetrahydrofuran (PTHF)-borate GPE with glass fiber to prepare a PTHF-based GPE (PTB@GF-GPE) for use in magnesium battery.47 This GPE exhibits reversible Mg plating/stripping behavior owing to its excellent interfacial compatibility and stability with magnesium metal. Moreover, Mo6S8/Mg battery using this PTB@GF-GPE delivers an ultra-high capacity retention (97.7% after 250 cycles at 0.5 C) at room temperature. More intriguingly, the battery using this electrolyte can also operate well over a wide operating temperature range (−20 °C−60 °C). The above-mentioned results prove again that in-situ polymerization strategy is universal and significantly improves the battery performance and safety issues of rechargeable batteries.
Conclusion and Challenges
In-situ polymerization of solid state lithium batteries have developed rapidly and witnessed great breakthroughs year by year. Herein, we have elaborated main progress and fundamental knowledge of in-situ polymerization of solid state lithium batteries from various perspectives. Aside from simplifying the preparation process, in-situ polymerization of polymer electrolytes can also form integrated interface to enhance interfacial compatibility, inhibit the dissolution of transition metal ions, suppress the growth of lithium dendrites, improve the cycle performance of silicon anodes, inhibit the shuttling effect of polysulfides and promote battery performance of post-lithium batteries. Indeed, in-situ polymerization of polymer electrolytes can be widely used in various rechargeable batteries such as solid state lithium batteries, solid state lithium-sulfur batteries, solid state lithium metal batteries, sodium batteries, lithium air batteries and magnesium batteries. Owing to the interfacial protection and integration structure, in-situ polymerization of solid state lithium batteries exhibited excellent interfacial compatibility and superior cycling stability.
Despite developments in solid state lithium batteries via in-situ polymerization, there remain many tough challenges:
- (1)At present, in-situ polymerization of polymer electrolytes still contain inflammable liquid electrolyte, which was detrimental to safety performance of batteries. Hence, using flame-retardant electrolyte as plasticizer may be an ideal solution.
- (2)Applied research on electrolytes is needed to develop developing high-energy solid state lithium batteries. Therefore, further improving electrochemical window and room-temperature ionic conductivity of solid state polymer electrolyte is very effective at promoting the comprehensive performance of solid state lithium batteries.
- (3)More systematic studies on various influencing factors (initiator activity, activation energy of initiator, half-value span of initiator, amount of initiator, polymerization temperature) to optimize battery performance should be intensively progressed so as to advance in-situ polymerization of solid state lithium batteries.
- (4)Selecting proper polymerization conditions and removing additional initiators. High polymerization temperature (i.e. 80 °C) deteriorates battery performance. Hence, selecting low-temperature active initiators (Cumene hydroperoxide/Iron Dichloride, Benzoyl Peroxide/N, N-dimethylaniline) is an ideal solution. Apart from high temperature, additional initiators (i.e. AIBN) can interact with alkali metal, resulting in the degradation of battery performance. Therefore, cationic/anionic polymerization initiated by lithium salts or alkali metal is also worth considering.
- (5)The remaining monomers in the in-situ GPEs will affect the battery performance. During the charge and discharge process, these residual monomers will decompose and deposit on the electrode surface, increase the electrode/electrolyte interfacial resistance and induce the degradation of cycle performance (especially at low temperature or high rate).48 Therefore, it is very important to choose appropriate polymerization conditions and reduce the residual amount of monomers. In addition, the remained solvents after polymerization can be quantitatively determined by gel permeation chromatography. The proportion of remained solvents (monomers) in polymer electrolytes is the ratio of the amount of monomers to the total amount of monomers and polymers.
- (6)There are still many challenges for scaling up the in-situ polymerization technology. For example, the heterogeneous heating often results in different degrees of polymerization, which is disadvantageous to the electrochemical properties. In addition, can the precursors sufficiently wet the separator and the electrodes with high active material loads? More importantly, most of the polymerization process may be accompanied by the generation of bubbles. How to ensure the uniformity of polymer electrolytes obtained by in-situ polymerization?
- (7)Most of the previously reported researches of in-situ polymerization mainly focus on the electrochemical performance and interfacial stability of polymer electrolytes, but ignore the evaluation of the polymerization level of in-situ generated polymer electrolytes. Actually, the appropriate polymerization level can be selected by balancing the electrochemical and mechanical properties of polymer electrolyte. Weight-averaged molecular weight (Mw), number-averaged molecular weight (Mn) and polydispersity index (Mw/Mn) of polymer electrolyte can be measured by gel permeation chromatography (GPC) to determine the polymerization level. Polydispersity index (Mw/Mn) indicates the uniformity of the polymerization. In addition, these three parameters are key to electrochemical performances of in-situ generated polymer electrolytes. Therefore, suitable polymerization conditions should be found to control the polymerization level of polymer electrolytes.
- (8)In terms of environmental pollution, we should choose the initiators without cyanogen group.
- (9)With respect to interfacial contact, the volume change of polymer electrolyte is also worthy of attention before and after in-situ polymerization.
- (10)The fundamental understanding of solid state lithium batteries via in-situ polymerization is insufficient. More advanced characterization techniques should be developed and used to further investigate the properties of interfaces and degradation mechanisms of solid state solid batteries.
Overall, it is clear that solid state lithium batteries via in-situ polymerization strategy can compete with lithium ion batteries using liquid electrolyte in some important respects, but they is still far from realizing the real utility of solid state lithium batteries. There are still many problems to be resolved when considering the practical application of in-situ polymerization of solid state lithium batteries. Many preparation processes of solid state lithium batteries can be realized in the laboratory, but it may not be suitable for mass production of actual application. There is much opportunity for research and development. Hence, innovations of in-situ polymerization methods are still needed for solid state lithium batteries in future. We believe that in-situ polymerization of solid state lithium batteries would ultimately become a reality through the unremitting scientific research.
Acknowledgments
This review was financially supported by National Natural Science Foundation of China (No. 51703236), National Science Fund for Distinguished Young Scholars (No. 51625204), National Key Research and Development Program of China (No. 2018YFB0104300).