Abstract
The durability of carbon-supported La-Mn-based perovskites for the oxygen reduction reaction in strong alkaline solutions was investigated. Carbon-supported perovskite-type oxide nanoparticles were prepared by using a reverse micelle method. The durability of the carbon-supported LaMnO3 nanoparticles was compared with that of carbon-supported LaMnO3 prepared by the mechanical mixing of LaMnO3 with the carbon support. As a result, the durability of the carbon-supported LaMnO3 nanoparticles was less than that of the carbon-supported LaMnO3 prepared by the mixing method due to a difference in the surface area of LaMnO3, which has an effect on the oxygen reduction reaction. In order to improve the durability of the carbon-supported LaMnO3 nanoparticles, Ca and Fe were substituted at the A-sites and B-sites of the perovskite lattice, respectively. As a result, it was found that the partial substitution of Ca and Fe is effective in improving the durability of LaMnO3 under cathodic polarization in strong alkaline solutions. In particular, the substitution of Ca at the A-site not only improved the durability of the oxide but also enhanced the oxygen reduction activity owing to an increase in the average valence state of the B-sites of the perovskite lattice.
Export citation and abstract BibTeX RIS
Gas diffusion electrodes (GDEs) are used as oxygen electrodes in metal–air batteries, fuel cells, and brine electrolysis. 1–5 GDEs are composed of a gas diffusion layer and a catalyst layer. The gas diffusion layer is fabricated with hydrophobic carbon and polytetrafluoroethylene (Teflon PTFE) in order to prevent the electrolyte from penetrating to the back side of the GDE. In the catalyst layer, the oxygen reduction reaction occurs at the point of contact where the electrolyte, the surface of the GDE, and oxygen meet. The oxygen reduction reaction causes high overvoltages unless there are carbon-supported electrocatalysts. In this respect, Pt, Ag, and its alloys have been studied as electrocatalysts for oxygen reduction reactions in alkaline media. 6–11 However, they are expensive and limited natural resources, and so low-cost and active electrocatalysts have been investigated by using various materials, e.g., metal oxides, 12–19 metal nitrides, 20, 21 metal sulfides, 22, 23 and metal chelates. 24, 25 Among them, Meadowcroft first pointed out that some perovskite-type oxides have high potentiality of oxygen reduction activity as compared with Pt-based noble metals. 26 Thus, many researchers have focused on these types of oxides as electrocatalysts for oxygen reduction. 27–29
To date, we have revealed that La-Mn-based perovskite-type oxides are candidate electrocatalysts because they have a high oxygen reduction activity. 30 Moreover, we have succeeded to disperse LaMnO3 nanoparticles on carbon support by using a reverse micelle (RM) method in order to gain catalytic reaction sites for the oxygen reduction reaction. As a result, the electrode potential of the carbon-supported LaMnO3 prepared by using the RM method was optimized to be equivalent to that of carbon-supported Pt nanoparticles. 31
For the practical use of La-Mn-based perovskite-type oxides as oxygen reduction catalysts, both the chemical and electrochemical durability are also important, aside from the oxygen reduction activity. Here, electrochemical durability means the stability against cathodic polarization of the GDE, because the oxygen reduction reaction shifts the electrode potential to negative relative to the equilibrium potential of the GDE. Thus far, the durability of perovskite-type oxides has been tested by Hyodo et al. 32 However, the mechanism of the decomposition of perovskite-type oxides was not discussed in detail in their report. In addition, the effect of the particle size, calcinations temperature, and the effect of partial substitution at the A- and B-sites of perovskite-type oxides were not investigated in their report. Generally, the chemical properties of perovskite-type oxides tend to change greatly by partial substitution at the A- and B-sites. Therefore, it can be possible to increase the durability of carbon-supported perovskite-type oxides prepared by partially substituting A- and B-sites of LaMnO3.
Therefore, in this study, we investigated the durability of La-Mn-based perovskite-type oxide nanoparticles on carbon support under the cathodic polarization in the strong alkaline solutions. The effects of the calcination temperature and partial substitution of Ca and Fe in the LaMnO3 lattice on the durability of the perovskite-type oxides were studied by means of x-ray diffraction (XRD), cyclic voltammogram, and quantitative analysis of the oxide remained on GDEs.
Experimental
Preparation of carbon-supported perovskite-type oxides
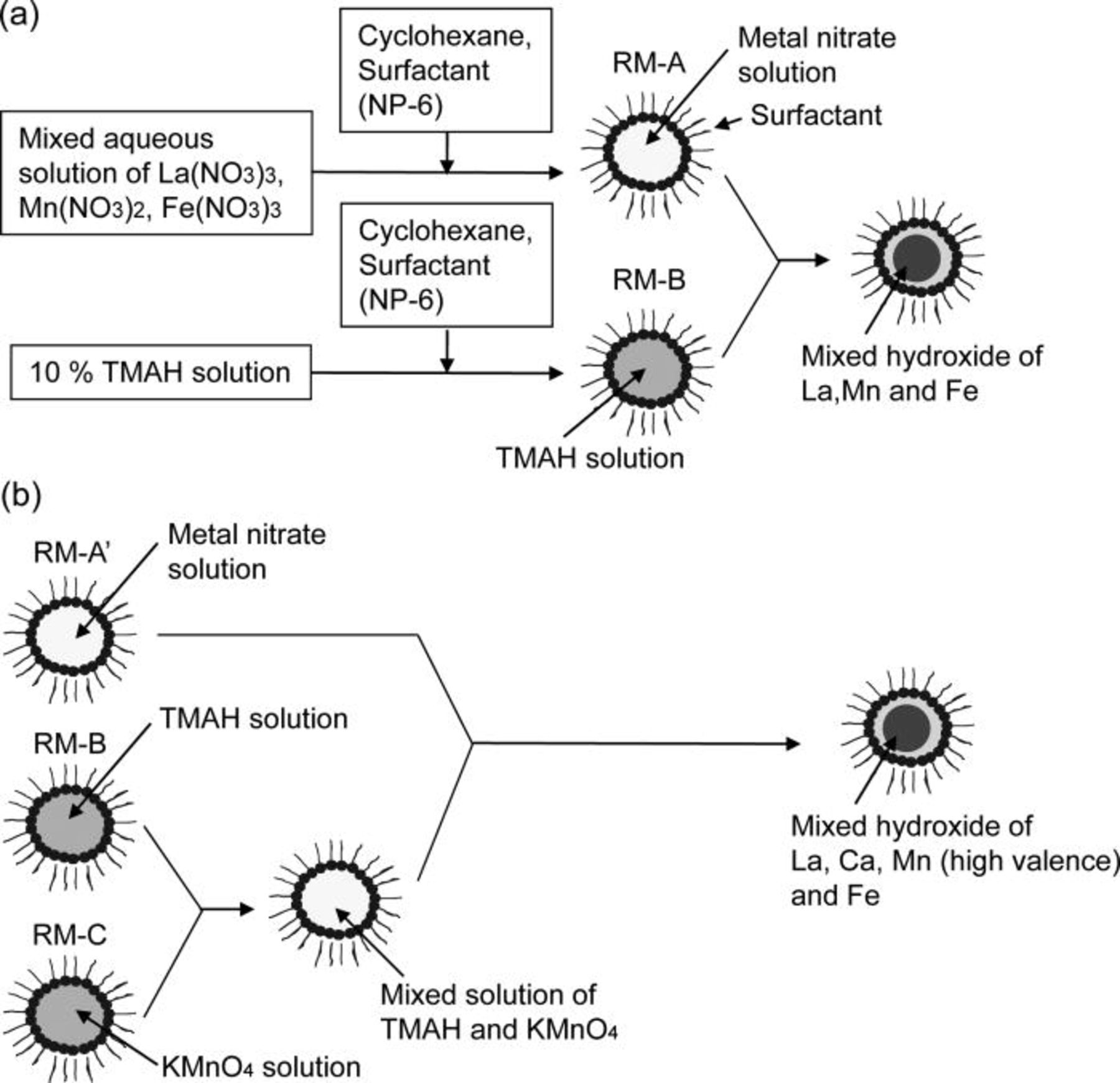
Figure 1a shows the procedure for preparing the precursor of the carbon-supported perovskite-type oxides (LaMnO3 and LaMn0.6Fe0.4O3) by using an RM method. In the first step, 1 ml of a mixed aqueous solution of a stoichiometric amount of La(NO3)3 (0.2 mmol), Mn(NO3)2 (0.12 mmol), and Fe(NO3)3 (0.08 mmol) was titrated into a mixed solution of cyclohexane (18 g) and hexaethyleneglycol nonylphenyl ether (NP-6, 9 g) in order to obtain the RM solution containing the metal nitrates (abbreviated as RM-A). Simultaneously, an RM solution containing a 10% tetramethylammonium hydroxide (abbreviated as TMAH) solution (abbreviated as RM-B) was prepared by mixing cylohexane (54 g), NP-6 (27 g), and 10% TMAH solution (3 ml). For RM-A and RM-B, the weight ratios of NP-6 to the aqueous solution and cyclohexane to NP-6 were fixed at 9 and 2, respectively. The RM-A and RM-B were then mixed together, leading to a brown RM solution. The brown RM solution appeared to contain mixed hydroxides of La, Mn, and Fe inside the RM.
Figure 1. Schematic diagram for the preparation of the precursor of (a) the carbon-supported LaMnO3 and La0.6Fe0.4O3 nanoparticles and (b) the carbon-supported La0.4Ca0.6Mn0.6Fe0.4O3 nanoparticles by using a reverse micelle method.
For preparing carbon-supported Ca- and Fe-partially substituted LaMnO3 (La0.4Ca0.6Mn0.6Fe0.4O3), manganese hydroxide of high valence was applied because some of the B-site cations in La0.4Ca0.6Mn0.6Fe0.4O3 should be transformed from trivalent to tetravalent in order to compensate the charge balance of the oxide. The procedure for preparing the precursor of the carbon-supported La0.4Ca0.6Mn0.6Fe0.4O3 using the RM method is shown in Fig. 1b. In the first step, 0.85 ml of a mixed aqueous solution of La(NO3)3 (0.08 mmol), Ca(NO3)2 (0.12 mmol), Mn(NO3)2 (0.06 mmol), and Fe(NO3)3 (0.08 mmol) was titrated into a mixed solution of cyclohexane (15.3 g) and NP-6 (7.65 g), in order to obtain the RM solution containing the metal nitrates (abbreviated as RM-A'). Simultaneously, the RM containing KMnO4 solution (RM-C) and the RM-B were prepared by mixing 0.3 ml of an aqueous solution of KMnO4 (0.06 mmol), cyclohexane (10 g), and NP-6 (2.7 g). The RM-B and RM-C were then mixed together, and an RM solution containing a mixed aqueous solution of TMAH and KMnO4 was obtained. The RM solution obtained was mixed with the RM-A', which was accompanied until a color of the RM solution changed in brown. It appeared that the mixed hydroxides of La, Ca, and Fe were obtained in the RM. At the same time, manganese hydroxides of high valence seemed to precipitate through the oxidation–reduction reaction between Mn2+ (Mn(NO3)2) and Mn7+ (KMnO4) in the RM.
Carbon black (EC600-JD, 1280 m2/g, Ketjen Black international Co.) was then added into the RM solutions containing the precursors of the perovskite-type oxides, which was obtained as shown in Figs. 1a and 1b. After ultrasonic blending was used to disperse the carbon black powder into the RM solutions, 200 ml of ethanol was added to the resultant RM solutions to break the RMs in order to load the metal hydroxides on the carbon black. The precipitates obtained were collected on the filter paper, and then the precipitates were washed with 500 ml of ethanol. After the precipitates were dried at 120°C for more than 6 h, the resultant powders were calcined at 550–700°C for 5 h in an N2 flow to prevent the carbon from combusting. Then, 200 mg of the carbon-supported perovskite-type oxides were dispersed in 30 ml of deionized water with a PTFE dispersion (32J, Daikin Kogyo Co., Ltd.) and 0.5 ml of n-butanol under vigorous stirring. The precipitates obtained were collected on the filter paper and dried at 120°C for more than 3 h. The powders for the catalyst layers were thus obtained. In the powders, the content of PTFE was fixed at 15 wt %.
Fabrication of GDEs
A mixture of acetylene black and 30 wt % PTFE was compressed into a thin sheet at 16 MPa on a Ni mesh (100 mesh) to act as a gas diffusion layer. The powder for the catalyst layer was then fabricated as a thin film on the gas diffusion layer under a pressure of 32 MPa. Finally, the catalyst layer and gas diffusion layer stacked on the Ni mesh were adhered to each other by a hot-press method at 365°C under a pressure of 64 MPa to furnish the GDEs.
Apparatus of the electrolysis and evaluation of the decomposed amount of perovskite-type oxide
The GDE obtained was welded with a Cu wire and mounted on the window of a PTFE cell, inside which 9 mol/l NaOH solution was placed as an electrolyte. The cell assembly was soaked in a water bath and held at 80°C. A Pt plate and an Hg/HgO electrode were used as a counter electrode and a reference electrode, respectively.
Cathodic polarization of the GDE for the oxygen reduction reaction was carried out using a potentiostat (HA-305G, Hokuto Denko Co., Ltd.) under O2 flow. When comparing the durability of oxides from different procedure, the cathodic polarization of GDE was carried out at a current density of 500 mA cm−2. When comparing the composition of oxides, the cathodic polarization of GDE was carried out at −100 mV versus Hg/HgO for 3 h.
The amount of decomposed perovskite-type oxides were quantified by means of an absorptiometric method. GDEs after the cathodic polarization were washed with deionized water and the catalyst layer was then removed. The catalyst layer was subsequently immersed in a 30% H2SO4 aqueous solution in order to dissolve the perovskite-type oxide remaining in the catalyst layer. After the remaining carbon black in the solution was removed with the filter paper, an excess amount of NaBiO3 was added to the filtrate in order to oxidize the Mn3+ or Mn4+ in the filtrate to  . Then, the absorbance of the solution at 525 nm was measured by means of a UV-Vis spectrometer (U-1000, Hitachi Ltd.) to quantify the molarity of
. Then, the absorbance of the solution at 525 nm was measured by means of a UV-Vis spectrometer (U-1000, Hitachi Ltd.) to quantify the molarity of  in the filtrate. Quartz cuvettes of path length 1 cm were used for the measurement. At first, the absorbance of calibration standards (2–10 μmol/l of KMnO4 solutions) at 525 nm was measured in order to prepare the standard curve for
in the filtrate. Quartz cuvettes of path length 1 cm were used for the measurement. At first, the absorbance of calibration standards (2–10 μmol/l of KMnO4 solutions) at 525 nm was measured in order to prepare the standard curve for  . Then the absorbance of the filtrate at 525 nm was measured. The molarity of the
. Then the absorbance of the filtrate at 525 nm was measured. The molarity of the  in the filtrate was determined by the obtained absorbance and the calibration curve. The amount of Mn in the perovskite and the decomposed amount of perovskite-type oxide were thus calculated from the molarity of
in the filtrate was determined by the obtained absorbance and the calibration curve. The amount of Mn in the perovskite and the decomposed amount of perovskite-type oxide were thus calculated from the molarity of  in the filtrate.
in the filtrate.
Results and Discussion
Effect of the preparation method and calcination temperature on the durability of LaMnO3
To investigate the durability of the carbon-supported LaMnO3 nanoparticles prepared by the RM method, the cathodic polarization of GDE was carried out at a current density of 500 mA cm−2 in 9 M NaOH at 80°C under an O2 flow, and the change of crystalline structure of LaMnO3 was investigated by means of an x-ray diffractometer with nickel-filtered Cu Kα (1.5418 Å) source (RINT2100, Rigaku Denki Co. Ltd., Japan). As a comparison, the carbon-supported LaMnO3 prepared by the mechanical mixing of LaMnO3 with the carbon support was investigated under the same conditions. In the sample of the mechanical mixing of LaMnO3 with the carbon support, LaMnO3 was prepared by the thermal decomposition of La, Mn-malic complex at 850°C.
33
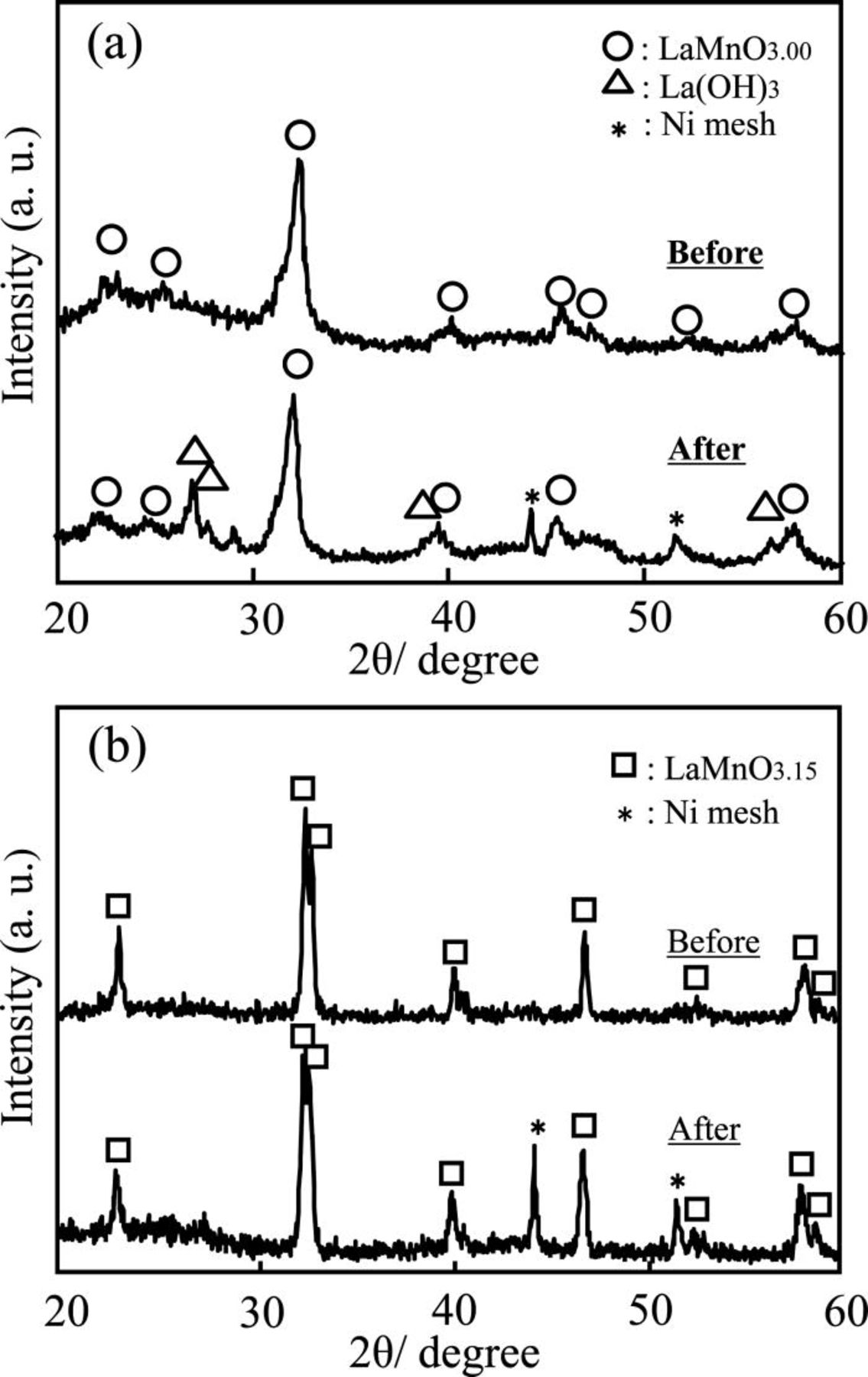
Figures 2a and 2b show changes in the XRD pattern of the GDE using carbon-supported LaMnO3 prepared by the RM method, and by the mechanical mixing of LaMnO3 with the carbon support, respectively. In the XRD pattern of the GDE using carbon-supported LaMnO3 prepared by the RM method, although there were no impurity phases except for the peaks of LaMnO3 before the cathodic polarization, we found that La(OH)3 coexisted with LaMnO3 after the cathodic polarization, as shown in Fig. 2a. This result indicates the decomposition of the LaMnO3 phase by the cathodic polarization. In addition to that, the change of coloring light blue was seen in the electrolyte after the cathodic polarization. This means that water-soluble metal ions were dissolved in the electrolyte. It seems that the dissolved ions are derived from Mn in the LaMnO3, because the La3+ ion cannot form water-soluble ions in the strong alkaline solutions.
33
Among many kinds of manganese compound,  (Mn7+),
(Mn7+),  (Mn6+),
(Mn6+),  (Mn5+), and
(Mn5+), and  (Mn2+) exist stably in the strong alkaline solution.
33, 34
In the LaMnO3 phase, it can be considered that valence state of Mn becomes not more than tetravalent under the cathodic polarization, because the valence state of Mn in the LaMnO3 phase seems to be trivalent or tetravalent. Therefore, the dissolved ion in the electrolyte seems to be the
(Mn2+) exist stably in the strong alkaline solution.
33, 34
In the LaMnO3 phase, it can be considered that valence state of Mn becomes not more than tetravalent under the cathodic polarization, because the valence state of Mn in the LaMnO3 phase seems to be trivalent or tetravalent. Therefore, the dissolved ion in the electrolyte seems to be the  ion, which is water-soluble divalent manganese ion.
35, 36
Taking into account the above considerations, it can be considered that Mn3+ or Mn4+ in LaMnO3 was reduced to Mn2+ by the cathodic polarization, and that the reduced Mn2+ ions were dissolved in the alkaline electrolyte.
ion, which is water-soluble divalent manganese ion.
35, 36
Taking into account the above considerations, it can be considered that Mn3+ or Mn4+ in LaMnO3 was reduced to Mn2+ by the cathodic polarization, and that the reduced Mn2+ ions were dissolved in the alkaline electrolyte.
Figure 2. XRD patterns of the GDE using carbon-supported LaMnO3 before and after polarization at 500 mA cm−2 for 3 h in 80°C 9 M NaOH solution under an O2 flow for (a) the carbon-supported LaMnO3 nanoparticles prepared by using a reverse micelle method and (b) the carbon-supported LaMnO3 prepared by the mechanical mixing of LaMnO3 with the carbon support. For the XRD pattern before the polarization, the carbon-supported LaMnO3 powder before the fabrication of the GDE was used.
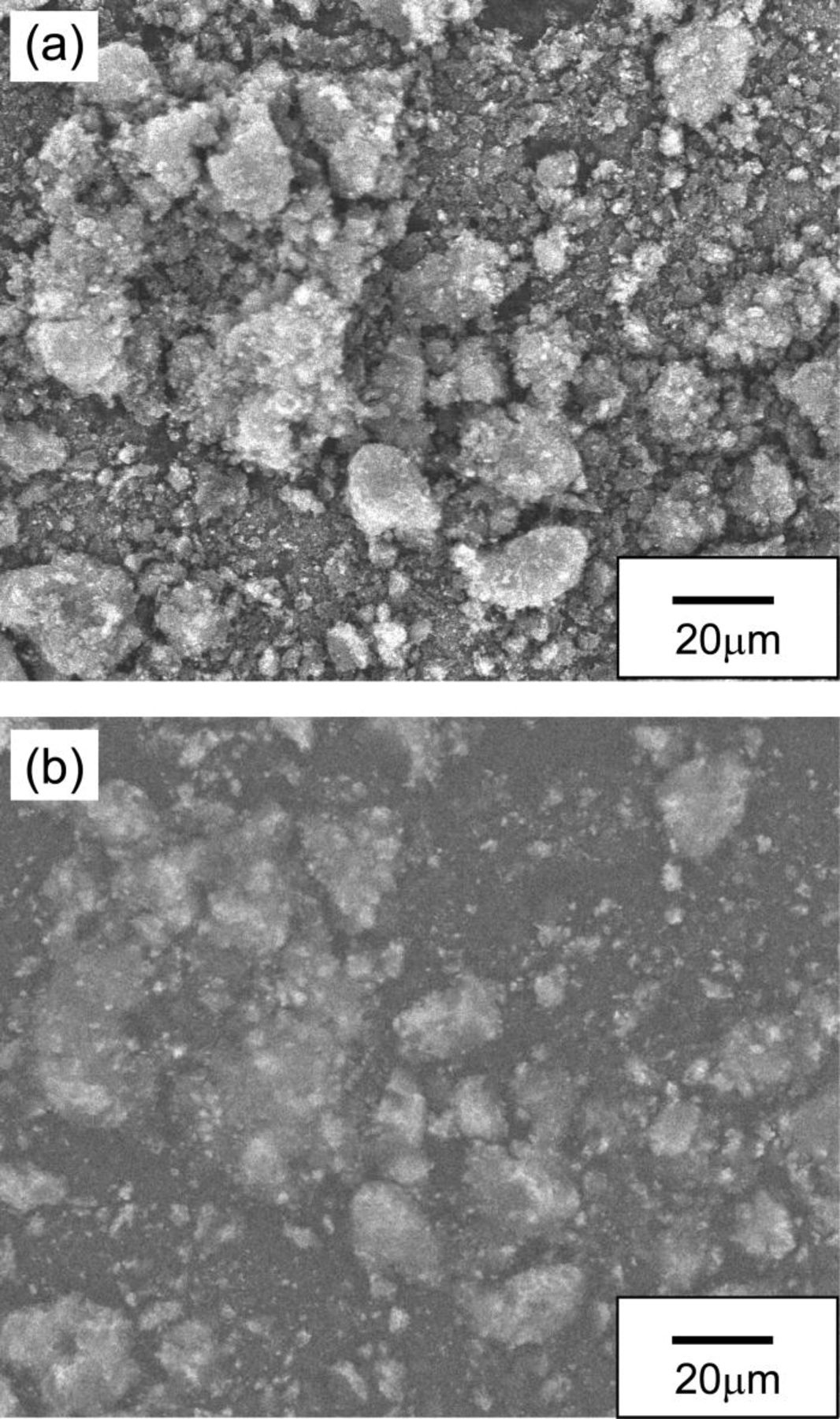
On the other hand, the catalyst prepared by the mechanical mixing of LaMnO3 with the carbon support was quite stable against the cathodic polarization in the strong alkaline solution, as shown in Fig. 2b. We propose that the difference in durability of the above two samples was caused by a difference in the dispersion of LaMnO3 on the carbon support. Therefore, we observed carbon-supported LaMnO3 powders using FE-SEM (JSM-6340F, JEOL Ltd., Japan). Figures 3 and 4 show the SEM images of carbon-supported LaMnO3 prepared by the RM method and by the mechanical mixing of LaMnO3 with the carbon support, respectively. In both Figs. 3 and 4, a secondary electron and a reflected electron images are arranged as (a) and (b), respectively. In the reflected electron images, bright spots and dark areas correspond to LaMnO3 particles and the carbon support, respectively, because the intensity of the reflected electrons tends to increase with increasing atomic number. In the carbon-supported LaMnO3 prepared by the mechanical mixing of LaMnO3 with the carbon support (Fig. 3), agglomeration of the LaMnO3 particles in the range of 1–20 μm can be seen in the reflected electron image. Conversely, in the carbon-supported LaMnO3 prepared by the RM method (Fig. 4), agglomerations of LaMnO3 particles are low in μm level, as compared to the carbon-supported LaMnO3 prepared by mixing the LaMnO3 with the carbon support. The difference in the dispersion of the LaMnO3 for the two samples is due to the loading process on the carbon support. In the carbon-supported LaMnO3 prepared by mixing of the LaMnO3 with the carbon support, it seemed that the LaMnO3 could not be dispersed on the carbon matrices because the LaMnO3 particles were already agglomerated before the loading. However, in the case of the carbon-supported LaMnO3 prepared by the RM method, precursors of the LaMnO3 were deposited on the carbon support in solution before calcination. Therefore, agglomeration of the LaMnO3 particles may be prevented by the carbon support. Taking into account the differences in the dispersion of LaMnO3 on the carbon support as shown in the SEM images, it appeared that the number of the oxygen reduction reaction sites on the LaMnO3 prepared by the RM method was larger than that of the carbon-supported LaMnO3 prepared by the mechanical mixing of LaMnO3 with the carbon support. Therefore, the amount of decomposition of LaMnO3 in the sample prepared by the RM method was larger than the amount in the samples of mixing agglomerated LaMnO3 with the carbon support.
Figure 3. FE-SEM image of the carbon-supported LaMnO3 prepared by the mechanical mixing of LaMnO3 with the carbon support: (a) secondary electron image and (b) reflected electron image.
Figure 4. FE-SEM image of the carbon-supported LaMnO3 nanoparticles prepared by using a reverse micelle method: (a) secondary electron image and (b) reflected electron image.
To investigate the durability of the carbon-supported LaMnO3 prepared by the RM method more thoroughly, the dependence of the durability on the calcination temperature was investigated. Table I summarizes the decomposed amount of carbon-supported LaMnO3 after polarization at −100 mV versus Hg/HgO for 3 h in 80°C 9 M NaOH solution. In the case of the carbon-supported LaMnO3 calcined at 550°C, we found that the color of the electrolyte after polarization was changed from transparent to light blue. The formation of a light blue solution seemed to indicate the presence of  ions, which are formed by the reduction of Mn3+ or Mn4+ in the LaMnO3 lattice to Mn2+.From the measurements of the amount of decomposed LaMnO3, we found that 45% of the LaMnO3 was decomposed after polarization at −100 mV for 3 h. However, as shown in Table I, the decomposed amount of the carbon-supported LaMnO3 decreased with an increase in the calcination temperature. It seemed that the LaMnO3 particles on the carbon support agglomerated more with an increase in the calcination temperature. Thus, the number of oxygen reduction reaction sites decreased with an increase in the calcination temperature, leading to a decrease in the amount of decomposed LaMnO3.
ions, which are formed by the reduction of Mn3+ or Mn4+ in the LaMnO3 lattice to Mn2+.From the measurements of the amount of decomposed LaMnO3, we found that 45% of the LaMnO3 was decomposed after polarization at −100 mV for 3 h. However, as shown in Table I, the decomposed amount of the carbon-supported LaMnO3 decreased with an increase in the calcination temperature. It seemed that the LaMnO3 particles on the carbon support agglomerated more with an increase in the calcination temperature. Thus, the number of oxygen reduction reaction sites decreased with an increase in the calcination temperature, leading to a decrease in the amount of decomposed LaMnO3.
Table I. Dependence of the amount of decomposed LaMnO3 after polarization on the calcination temperature of the carbon-supported LaMnO3. Polarization was performed at −100 mV vs Hg/HgO for 3 h at 80°C in 9 M NaOH solution under O2 flow.
| Calcination temperature of LaMnO3/°C | 550 | 600 | 650 | 700 |
|---|---|---|---|---|
| Amount of the decomposed LaMnO3/mol % | 45 | 18 | 15 | 12 |
Effect of the substitution of Ca and Fe on the stability of LaMnO3
As mentioned in the previous section, the durability of the carbon-supported LaMnO3 prepared by the RM method against cathodic polarization increased with an increase in the calcination temperature. However, increasing the calcination temperature of LaMnO3 is not desirable, because high calcination temperatures cause agglomeration of the LaMnO3 particles, leading to a decrease in the oxygen reduction activity. Therefore we aimed to improve the durability of the carbon-supported LaMnO3 by not using a high calcination temperature, but through the investigation of the composition of the perovskite-type oxide.
As for the stability of LaBO3 (B = V, Cr, Mn, Fe, Co, Ni) against the reducing of B3+/4+ to B2+,Nakamura et al. investigated under low oxygen partial pressure at high temperature using a TG/DTA and powder x-ray diffractions. 37 As a result, they found that LaCrO3 and LaFeO3 are more stable than LaMnO3 under reducing atmosphere. Therefore, we deduced that LaCrO3 and LaFeO3 are more stable than LaMnO3 under the cathodic polarization in the strong alkaline solution. Among LaCrO3 and LaFeO3, LaCrO3 is difficult to prepare using the RM method, because Cr(OH)3 is dissolved in the strong alkaline solution. 34 Therefore, we choose LaFeO3 as a candidate for new component for oxygen reduction catalyst.
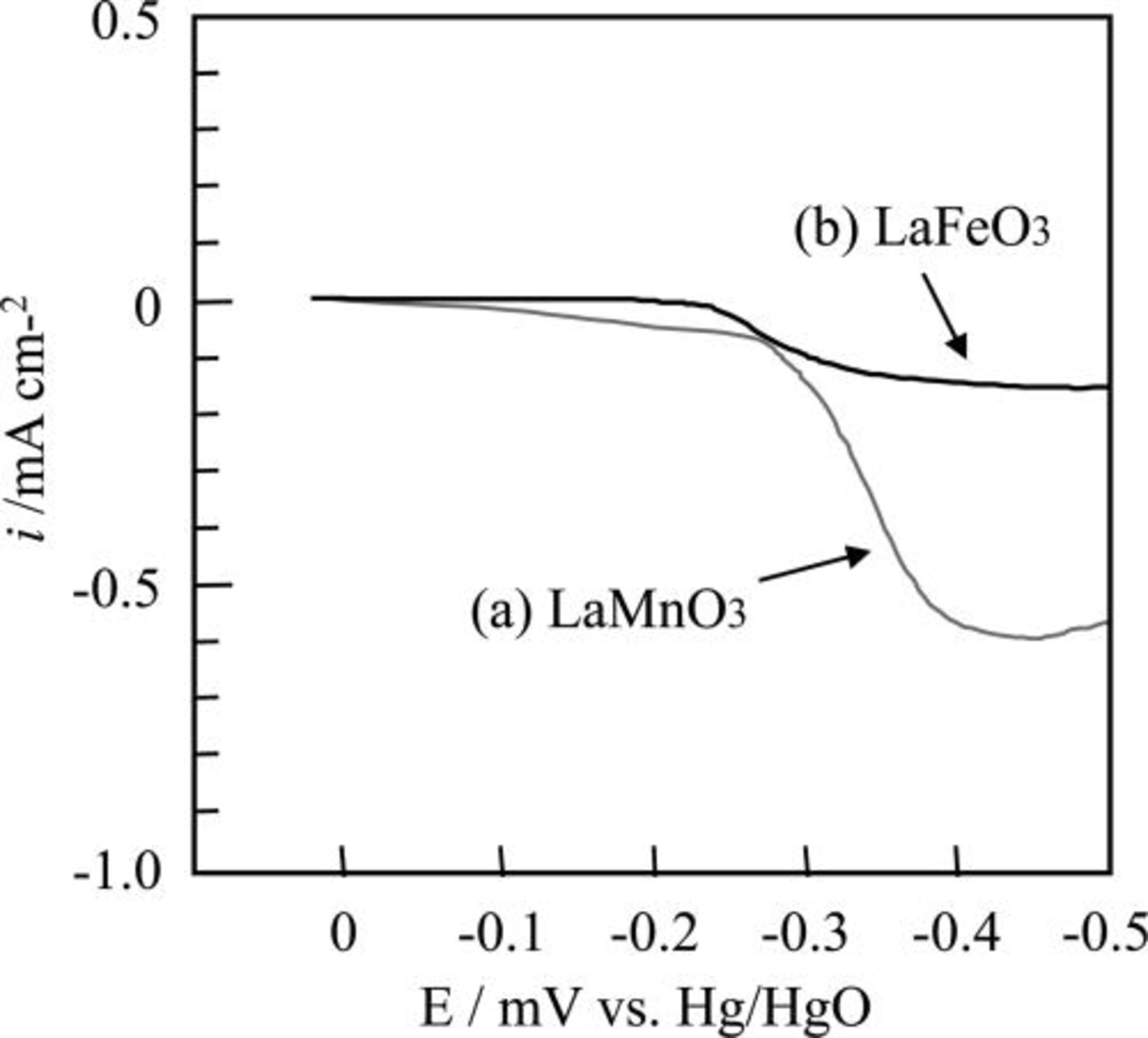
At first, difference of the durability under cathodic polarization between LaMnO3 and LaFeO3 was investigated. To evaluate the durability of LaBO3, a linear sweep voltammetry (LSV) for the LaMnO3 and LaFeO3 disks was carried out at 80°C in 9 M NaOH aqueous solution under N2 bubbling. The sweep rate of LSV measurements was −2 mV/s. The sample disk was fabricated by sintering LaBO3 pellets at 1000°C for 5 h in air. Figure 5 shows the linear sweep voltammetry curves for the LaMnO3 and the LaFeO3 disks. In Fig. 5, negative current denotes the reduction of perovskite-type oxides because dissolved oxygen in the electrolyte was removed by the N2 bubbling. For the LaMnO3 disk, a small negative current was observed in the range of 0−0.3 V versus Hg/HgO. And a large negative current was observed at the potential of lower than −0.3 V versus Hg/HgO. This result indicates that the LaMnO3 can be reduced slowly even at the small cathodic polarization. On the contrary, in the case of the LaFeO3 disk, a negative current was not seen in the range of 0–0.35V versus Hg/HgO. Therefore we presumed that the partial substitution of Fe at the B-sites of LaMnO3 could stabilize the perovskite phase against cathodic polarization.
Figure 5. The linear sweep voltammogram of (a) the LaMnO3 and (b) the LaFeO3 disks. Sweep rate was −2mV/s.
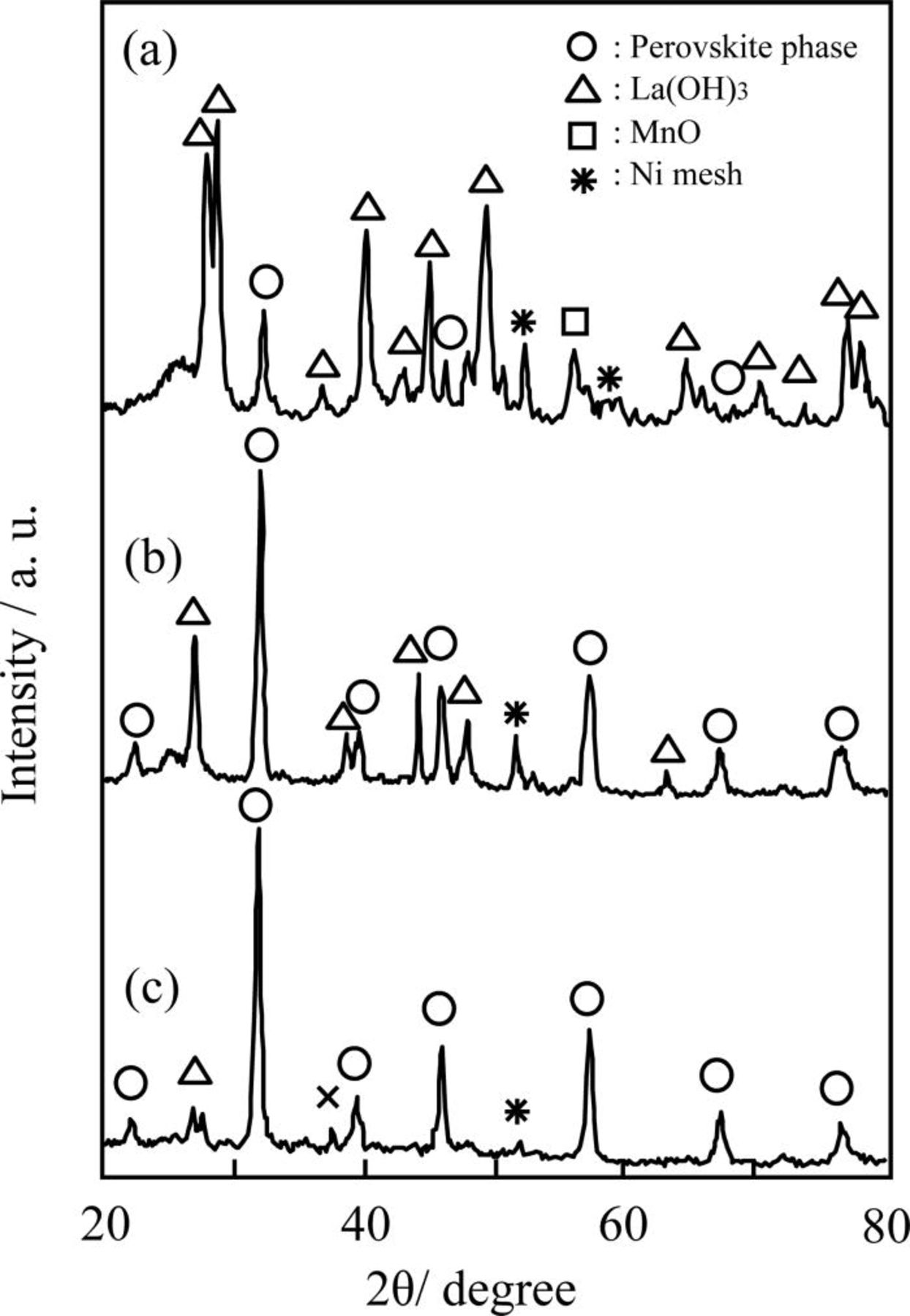
Thus, carbon-supported LaMn0.6Fe0.4O3 was prepared by using the RM method, and its durability against cathodic polarization was examined. In addition, it has been reported that the partial substitution of Ca or Sr at the A-sites of perovskite-type oxides improves their oxygen reduction activity. 28 The Ca- or Sr-partial substitution increases the valence state of the B-sites to compensate the charge valance of the perovskite phase. Such an increase in the valence state of the B-sites has been confirmed by means of the XPS measurement. 38, 39 An increase in the valence state of the B-sites could possibly increase the durability of La-Mn-based perovskite-type oxides against the cathodic polarization. Thus, the durability of La0.4Ca0.6Mn0.6Fe0.4O3 was also investigated. Figure 6 shows the XRD patterns of the GDE using carbon-supported LaMnO3, LaMn0.6Fe0.4O3 and La0.4Ca0.6Mn0.6Fe0.4O3 after polarization at −100 mV versus Hg/HgO for 120 h at 80°C in 9 M NaOH solution. Furthermore, Table II summarizes the average valence state of the B-sites of the oxides, the amount of decomposed perovskite-type oxides after polarization for 120 h, and the current density under polarization at −100 mV versus Hg/HgO. The average valence state of the B-sites of the perovskite-type oxides was quantified by iodometric titration. 40 The current density summarized in Table II indicates the oxygen reduction activity of the carbon-supported oxides. In each specimen, carbon-supported oxides (30 wt %) were prepared using the RM method, and was calcined at 700°C. In the case of carbon-supported LaMnO3, the formation of a large amount of La(OH)3 was confirmed by the XRD pattern (Fig. 6a). Such formation of La(OH)3 appeared to be caused by the decomposition of LaMnO3 under cathodic polarization. The amount of decomposed LaMnO3 was quantified as 65 mol %.Conversely, in the case of carbon-supported LaMn0.6Fe0.4O3, the relative peak intensity of the La(OH)3 to the perovskite-type oxide in the XRD patterns in Fig. 6 was small when compared with the carbon-supported LaMnO3. In addition, the decomposed amount of oxide was significantly decreased as compared to the carbon-supported LaMnO3. Such phenomena indicate that the partial substitution of Fe at the B-sites of LaMnO3 was effective at increasing the electrochemical durability against the cathodic polarization in the strong alkaline solutions. However, we found that the oxygen reduction activity of the carbon-supported LaMn0.6Fe0.4O3 was inferior to carbon-supported LaMnO3, as shown in the current density at −100 mV versus Hg/HgO in Table II. Thus far, it has been reported that LaFeO3 is less active than LaMnO3. 32, 41 In addition, it has been claimed that the catalytic active sites of perovskite-type oxides for oxygen reduction are B-site cations. 42 Therefore, one can say that Fe cations in the perovskite-type oxides are less active than Mn cations in the perovskite-type oxides. Additionally, the oxygen reduction activity of carbon-supported LaMn0.6Fe0.4O3 was less than that of carbon-supported LaMnO3.
Figure 6. XRD patterns of GDEs after polarization for the carbon-supported (a) LaMnO3, (b) LaMn0.6Mn0.4O3, and (c) La0.4Ca0.6Mn0.6Fe0.4O3. The polarization was performed at −100 mV vs Hg/HgO for 120 h in 80°C 9 M NaOH solution under O2 flow.
Table II. Average valence state of B-sites and decomposition amount of oxides after polarization for the carbon-supported LaMnO3, LaMn0.6Fe0.4O3, and La0.4Ca0.6Mn0.6Fe0.4O3. The polarization was performed at −100 mV vs Hg/HgO for 120 h at 80°C in 9 M NaOH solution under O2 flow.
| Average valence of B-site | Decomposition amount of oxide/mol % | Current density under polarization (−100 mV vs Hg/HgO)/mA cm−2 | |
|---|---|---|---|
| LaMnO3 | 3.23 | 65 | 700 |
| LaMn0.6Fe0.4O3 | 3.24 | 17 | 580 |
| La0.4Ca0.6Mn0.6Fe0.4O3 | 3.58 | 5.5 | 1060 |
When Ca was partially substituted at 60% of the A-sites of LaMn0.6Fe0.4O3 (La0.4Ca0.6Mn0.6Fe0.4O3), the average valence state of the B-sites was increased by about 35% as compared with LaMnO3 and LaMn0.6Fe0.4O3 (Table II). When we compared the oxygen reduction activity of carbon-supported La0.4Ca0.6Mn0.6Fe0.4O3 with those of carbon-supported LaMnO3 and LaMn0.6Fe0.4O3, we found that carbon-supported La0.4Ca0.6Mn0.6Fe0.4O3 was far superior to the others. Such a tendency is in good agreement with the tendencies previously reported.
43
The oxygen reduction activity of the La-Mn-based perovskite oxides seem to depend on the content of tetravalent cations in the B-sites. However, the correlation between the content of the tetravelent cations in the B-sites and the oxygen reduction activity has not yet been elucidated. Further investigations are required in this area. In terms of durability of the oxide La0.4Ca0.6Mn0.6Fe0.4O3, the relative peak intensity of La(OH)3 to the perovskite-type oxide of was far reduced when compared with the carbon-supported LaMn0.6Fe0.4O3, as shown in Fig. 6. Furthermore, the decomposed amount on the oxides was restrained up to 5.5%.These results indicated that the high valence state of the B-sites, caused by the substitution of Ca at the A-sites, was effective at increasing the durability against the cathodic polarization in the strong alkaline solutions. As shown above, Mn3+ or Fe3+ cation in the perovskite lattice tend to be reduced to water-soluble species such as  (Mn2+) or
(Mn2+) or  (Fe2+) under the cathodic polarization. However, when the B-sites of LaMn0.6Fe0.4O3 are shifted to high valence states by partially substituting Ca at the A-sites, it appeared that B-site cation cannot be easily reduced to water-soluble species due to the higher valence state of the B-sites as compared with LaMn0.6Fe0.4O3. From the above results, we can state that the partial substitution of Ca at the A-sites of perovskite-type oxides is effective at increasing both the oxygen reduction activity and durability against the cathodic polarization.
(Fe2+) under the cathodic polarization. However, when the B-sites of LaMn0.6Fe0.4O3 are shifted to high valence states by partially substituting Ca at the A-sites, it appeared that B-site cation cannot be easily reduced to water-soluble species due to the higher valence state of the B-sites as compared with LaMn0.6Fe0.4O3. From the above results, we can state that the partial substitution of Ca at the A-sites of perovskite-type oxides is effective at increasing both the oxygen reduction activity and durability against the cathodic polarization.
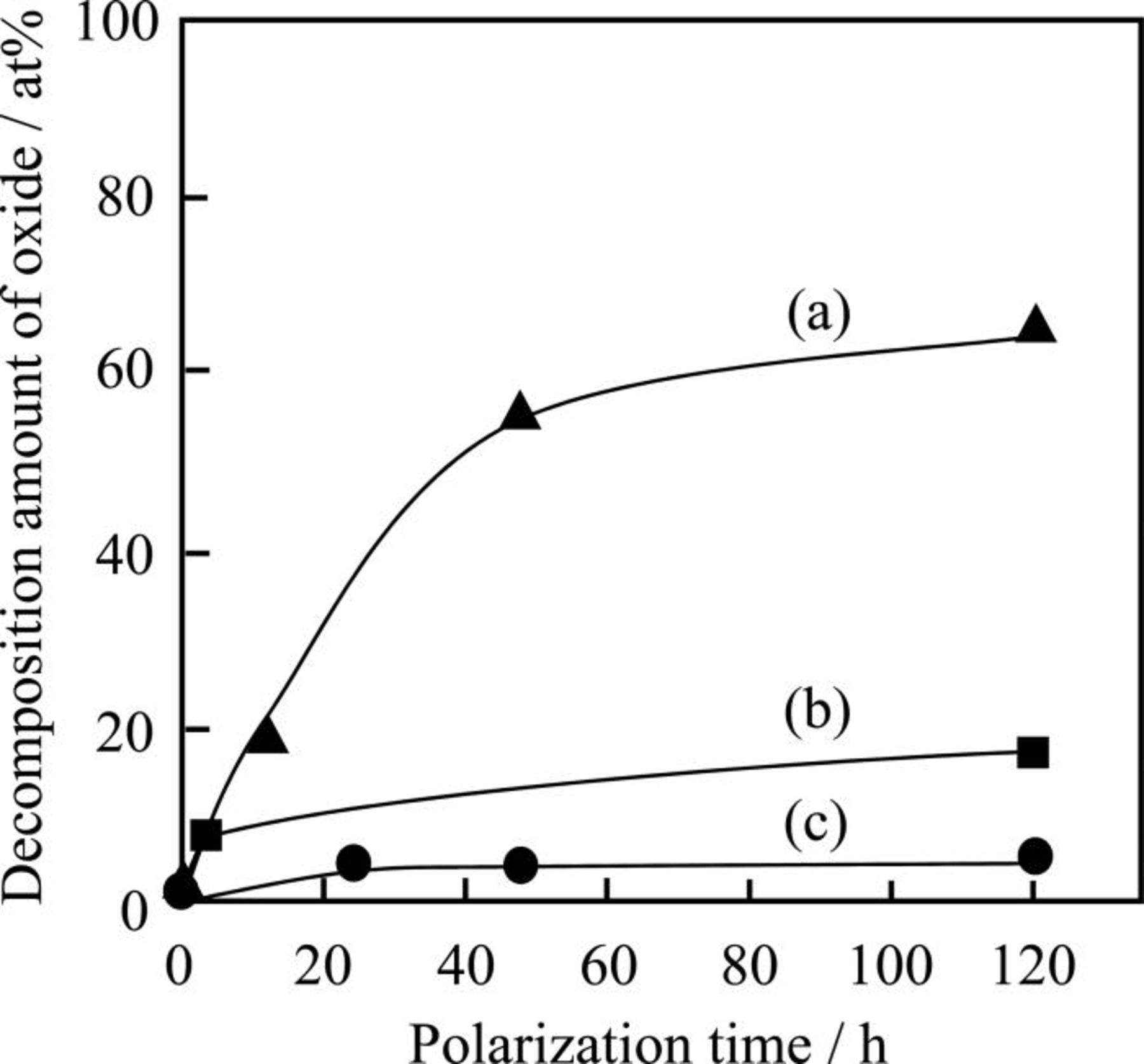
In order to investigate the dynamics of the decomposition of oxides, the decomposition amount of oxides were measured after various polarization terms. Figure 7 shows the dependence of the decomposition amount of oxide for the carbon-supported LaMnO3, LaMn0.6Fe0.4O3, and La0.4Ca0.6Mn0.6Fe0.4O3 on the polarization term. It was remarkable for the carbon-supported LaMnO3 that the decomposition of oxide is fast in the early stage, and that the decomposition of oxide slowed gradually. When LaMnO3 is decomposed under the cathodic polarization, LaMnO3 can be decomposed to water-soluble  and La(OH)3. Although
and La(OH)3. Although  is soluble to the electrolyte, La(OH)3 is indissoluble to the strong alkaline solution. Therefore, La(OH)3 was deposited on the LaMnO3 particles which have not been decomposed, leading to the eclipse of LaMnO3 surface. For the similar reasons, the decomposition of carbon-supported LaMn0.6Fe0.4O3 was decomposed gradually. On the contrary, La0.4Ca0.6Mn0.6Fe0.4O3 was fairly stable after the polarization for 120 h because La0.4Ca0.6Mn0.6Fe0.4O3 is durable against the cathodic polarization as mentioned above.
is soluble to the electrolyte, La(OH)3 is indissoluble to the strong alkaline solution. Therefore, La(OH)3 was deposited on the LaMnO3 particles which have not been decomposed, leading to the eclipse of LaMnO3 surface. For the similar reasons, the decomposition of carbon-supported LaMn0.6Fe0.4O3 was decomposed gradually. On the contrary, La0.4Ca0.6Mn0.6Fe0.4O3 was fairly stable after the polarization for 120 h because La0.4Ca0.6Mn0.6Fe0.4O3 is durable against the cathodic polarization as mentioned above.
Figure 7. The dependence of the decomposition amount of oxide for the carbon-supported (a) LaMnO3, (b) LaMn0.6Fe0.4O3, and (c) La0.4Ca0.6Mn0.6Fe0.4O3 on the polarization term.
Conclusion
A carbon-supported La-Mn-based perovskite-type oxide for the catalyst layer of gas diffusion electrodes was prepared by using a reverse micelle method. The durability of the carbon-supported perovskite-type oxides was tested in the strong alkaline media under the cathodic polarization. When we compared the durability of the carbon-supported oxide nanoparticles with that of a mixture of agglomerated oxides and carbon support, we found that the durability of the carbon-supported oxide nanoparticles was less than that of the composite due to differences in the active sites for oxygen reduction. The partial substitution of Fe at the B-sites of LaMnO3 was effective in stabilizing the LaMnO3 nanoparticles. In addition, the partial substitution of Ca at the A-sites of LaMn0.6Fe0.4O3 increased the average valence state of the B-sites, leading to improvements in both the durability and the oxygen reduction activity of the carbon-supported perovskite-type oxides.
Kyushu University assisted in meeting the publication costs of this article.