Abstract
Dealloyed Pt–Cu alloy nanoparticles are active oxygen reduction electrocatalysts; they are formed from Cu-rich alloy precursors during a selective Cu atom dissolution (dealloying) process. The surface of Cu-rich particle precursors is prone to oxidation under ambient air conditions, which may critically affect the aging behavior of the precursors. Here, we present a systematic stability and aging study of a carbon-supported  alloy nanoparticle catalyst precursor. We study the impact of the aging of the catalyst material on its electrocatalytic performance for the oxygen reduction reaction (ORR) after dealloying. We obtain a practical insight into the electrochemical behavior of the materials in the formats of powders, inks, and films. Our studies suggest that the Pt–Cu precursors show a stable catalytic performance when aged as dry powders in air. Ink samples, however, reach their maximum ORR activity of up to
alloy nanoparticle catalyst precursor. We study the impact of the aging of the catalyst material on its electrocatalytic performance for the oxygen reduction reaction (ORR) after dealloying. We obtain a practical insight into the electrochemical behavior of the materials in the formats of powders, inks, and films. Our studies suggest that the Pt–Cu precursors show a stable catalytic performance when aged as dry powders in air. Ink samples, however, reach their maximum ORR activity of up to  with aging for 24–48 h after which they deteriorated in performance. Finally, catalyst thin films were the most sensitive to aging in air and generally deteriorated rapidly after just one day. Our results provide practical insights and guidelines regarding the stability and handling of the nanoparticle catalyst powder.
with aging for 24–48 h after which they deteriorated in performance. Finally, catalyst thin films were the most sensitive to aging in air and generally deteriorated rapidly after just one day. Our results provide practical insights and guidelines regarding the stability and handling of the nanoparticle catalyst powder.
Export citation and abstract BibTeX RIS
The study of Pt and Pt-alloy systems as electrocatalysts for use in polymer-electrolyte membrane fuel cells (PEMFCs) has generated much interest in both the scientific and industrial communities.1, 2 This is due to the high efficiency and low pollution that fuel cell promises as an alternative energy source. To understand the catalytic activity and stability of the fuel cell catalytic reactions on Pt and Pt alloys, these catalysts were studied in the form of single-crystal, polycrystalline, and carbon-supported nanoparticles.3, 4
Pt and Pt-alloy single-crystal electrocatalysts for the ORR were studied in much detail by Markovic and co-workers.5–10 This team reported catalytic activity enhancements of Pt alloy single crystals of up to nine times compared to the corresponding pure Pt crystal surface. However, a severe shortcoming of single-crystal fuel cell electrocatalysts is the requirement for a perfectly well-defined and clean surface. The sensitivity and susceptibility of these clean surfaces to contamination from air require that experiments be conducted either in ultrahigh vacuum or inert atmosphere (usually Ar or  ).6, 11–14
).6, 11–14
Pt and Pt-alloy polycrystalline surfaces provide a bridge between single-crystal surfaces and high surface area catalyst formats. Polycrystalline surfaces consist of an ensemble of surfaces and each attribute to the final electrochemical activity measured. In addition, the surface composition of the polycrystalline can be tuned accurately by adjusting the sputtering rate during the synthesis process. Toda et al. had reported detailed studies of Pt–M ( , Ni, Co) systems,15, 16 while Stamenković et al. presented the studies of Pt-skin and Pt-skeleton polycrystalline materials.17 The ORR activities of the polycrystalline surfaces were lower than those of selected single-crystal surfaces, yet were again clearly superior to high surface area catalysts due to the so-called "particle size effect," which results from a lower amount of coordinately undersaturated surface atoms on extended surfaces.18–21 The polycrystalline surfaces are susceptible to contamination in air and again require surface cleaning, preparation, and electrochemical characterization to be performed in a controlled environment. In addition, due to the nature of the catalyst surface, a mass-based activity cannot be obtained.22
, Ni, Co) systems,15, 16 while Stamenković et al. presented the studies of Pt-skin and Pt-skeleton polycrystalline materials.17 The ORR activities of the polycrystalline surfaces were lower than those of selected single-crystal surfaces, yet were again clearly superior to high surface area catalysts due to the so-called "particle size effect," which results from a lower amount of coordinately undersaturated surface atoms on extended surfaces.18–21 The polycrystalline surfaces are susceptible to contamination in air and again require surface cleaning, preparation, and electrochemical characterization to be performed in a controlled environment. In addition, due to the nature of the catalyst surface, a mass-based activity cannot be obtained.22
Finally, carbon-supported high surface area Pt and Pt-alloy electrocatalysts (Pt–M/C) have been one of the most promising methods of implementing electrocatalysts in PEMFC and for enhancing ORR activities.6, 16, 22–26 Carbon-supported Pt alloy systems are reported to exhibit catalytic activities of two to four times that of carbon-supported pure Pt (Pt/C).23, 24, 27
Recent studies on nanostructured catalyst concepts such as Pt monolayer11, 28 catalysts and core-shell nanoparticles29, 30 reported activity enhancements of up to six times compared to pure Pt nanoparticles.31 The alloy electrocatalysts are also more stable than the carbon-supported Pt, and the reduced Pt loading also diminished catalyst costs in PEMFCs.32–34
In nanoparticle electrocatalyst studies, researchers do not commonly address the storage, treatment, and exposure to ambient conditions of the electrocatalysts powders. This is likely because the electrochemical pretreatment after the electrocatalysts is cast into a catalyst thin film is typically thought to remove all surface contaminants and to create a reproducible particle surface and, hence, catalyst performance. However, little work has been reported to study this issue, especially in Pt alloy systems. It is reasonable to assume that surface contaminants and metal oxide formation impact surface catalytic performance of alloy nanoparticles despite electrochemical pretreatment protocols.35, 36 This is especially relevant for alloy electrocatalysts, which are rich in base metals, such as the Cu-rich 
 precursors of dealloyed Pt alloy ORR catalysts.30, 32–34, 37–43
precursors of dealloyed Pt alloy ORR catalysts.30, 32–34, 37–43
The goal of the present work is to elucidate the stability and aging behavior of  alloy nanoparticle precursor materials that are the starting point for the synthesis of the active dealloyed Pt–Cu nanoparticles ORR electrocatalysts. Synthesized using an impregnation–freeze–drying–annealing route, the materials are systematically aged in air in the form of as-prepared powders, aqueous inks, and dried thin films and tested for their ORR activities in acidic media. Our results highlight unexpected aspects of the relation between the powder air aging and the associated catalytic performance of a Pt alloy nanoparticle catalyst. Our findings provide practical guidelines to optimize the performance of a catalyst powder, ink, and film and are relevant to other catalyst systems.
alloy nanoparticle precursor materials that are the starting point for the synthesis of the active dealloyed Pt–Cu nanoparticles ORR electrocatalysts. Synthesized using an impregnation–freeze–drying–annealing route, the materials are systematically aged in air in the form of as-prepared powders, aqueous inks, and dried thin films and tested for their ORR activities in acidic media. Our results highlight unexpected aspects of the relation between the powder air aging and the associated catalytic performance of a Pt alloy nanoparticle catalyst. Our findings provide practical guidelines to optimize the performance of a catalyst powder, ink, and film and are relevant to other catalyst systems.
Experimental
Synthesis of the Pt–Cu alloy nanoparticle electrocatalyst precursor
For the entire study, a single homogeneous batch of a carbon-supported  (Pt–Cu/C) material was prepared, which served as the precursor of the dealloyed active phase of the electrocatalyst. The precursor was synthesized using a liquid metal salt impregnation method followed by freeze-drying and thermal annealing. This method had been applied repeatedly for the synthesis of binary30, 40, 41, 44–46 and ternary42 Pt alloys. In short, the solid Cu precursor [
(Pt–Cu/C) material was prepared, which served as the precursor of the dealloyed active phase of the electrocatalyst. The precursor was synthesized using a liquid metal salt impregnation method followed by freeze-drying and thermal annealing. This method had been applied repeatedly for the synthesis of binary30, 40, 41, 44–46 and ternary42 Pt alloys. In short, the solid Cu precursor [ , Sigma Aldrich] was dissolved in
, Sigma Aldrich] was dissolved in  deionized water (millipore) and added to commercially purchased, high surface area, carbon-supported Pt nanoparticles (catalyst part no. TEC10E30E supplied by Tanaka Kikinzoku Inc., Japan). The mixture was then ultrasonicated (Branson Sonifier 150 with sonifier horn) and frozen in liquid nitrogen. The frozen sample was then freeze-dried (Labconco, 12 L tray dryer, 0.05 mbar) in vacuum overnight at room temperature. The powders obtained were then annealed (Lindberg Blue flow furnace) to maximum temperatures of
deionized water (millipore) and added to commercially purchased, high surface area, carbon-supported Pt nanoparticles (catalyst part no. TEC10E30E supplied by Tanaka Kikinzoku Inc., Japan). The mixture was then ultrasonicated (Branson Sonifier 150 with sonifier horn) and frozen in liquid nitrogen. The frozen sample was then freeze-dried (Labconco, 12 L tray dryer, 0.05 mbar) in vacuum overnight at room temperature. The powders obtained were then annealed (Lindberg Blue flow furnace) to maximum temperatures of  for 7 h under a 4% hydrogen atmosphere (argon balance) and slowly cooled down to room temperature by natural convection. The catalyst precursor used for this study has a composition of
for 7 h under a 4% hydrogen atmosphere (argon balance) and slowly cooled down to room temperature by natural convection. The catalyst precursor used for this study has a composition of  and had a final Pt weight loading at around 22 wt%. This precursor would thus be referred to as "
and had a final Pt weight loading at around 22 wt%. This precursor would thus be referred to as " (800/7)." The precursor powder was generally stored under flowing dry nitrogen.
(800/7)." The precursor powder was generally stored under flowing dry nitrogen.
Catalyst ink formulation and catalyst thin-film preparation
A catalyst ink was prepared by mixing weighed amounts of the catalyst precursor powder in 10 mL of a degassed  aqueous solution containing 5 wt% Nafion (Sigma Aldrich) solution and 2-propanol (Sigma Aldrich). The suspension was ultrasonicated for 15 min at room temperature (Branson 150) before
aqueous solution containing 5 wt% Nafion (Sigma Aldrich) solution and 2-propanol (Sigma Aldrich). The suspension was ultrasonicated for 15 min at room temperature (Branson 150) before  of aliquot was dispensed onto the glassy carbon (GC) surface of a rotating disk electrode (RDE) (PINE Instrument). The ink was then dried for 10 min in a
of aliquot was dispensed onto the glassy carbon (GC) surface of a rotating disk electrode (RDE) (PINE Instrument). The ink was then dried for 10 min in a  atmosphere resulting in a thin film on the GC surface. Film thicknesses were estimated to range between 0.1 and
atmosphere resulting in a thin film on the GC surface. Film thicknesses were estimated to range between 0.1 and  , using the dry density of Nafion and neglecting the volume of the catalyst. The dried films were kept under nitrogen until the electrochemical characterization commenced.
, using the dry density of Nafion and neglecting the volume of the catalyst. The dried films were kept under nitrogen until the electrochemical characterization commenced.
Electrochemical measurements
The electrochemical cell used was a custom-made, three-compartment glass cell. The working electrode was commercially obtained with a 5 mm fixed diameter GC surface. The counter electrode was a piece of Pt gauze (Sigma Aldrich). The reference electrode was a mercury–mercurous sulfate electrode (Princeton Applied Research, AMETEK) held in place by a Luggin–Haber capillary. All electrode potentials reported were converted into the reversible hydrogen electrode (RHE) scale. A commercial dual-channel potentiostat (BioLogic) and a rotator (PINE Instrument) were used to conduct the rotating disk experiment. The electrolyte used was 70% redistilled  (Sigma Aldrich) and diluted to a 0.1 M concentration. All measurements were conducted at room temperature, and the working electrode was immersed into the electrolyte under potential control at 0.05 V until actual measurements commenced. Cyclic voltammetry (CV) was performed in a deaerated electrolyte under
(Sigma Aldrich) and diluted to a 0.1 M concentration. All measurements were conducted at room temperature, and the working electrode was immersed into the electrolyte under potential control at 0.05 V until actual measurements commenced. Cyclic voltammetry (CV) was performed in a deaerated electrolyte under  atmosphere. The voltammetric response of the electrocatalysts was first measured during the initial three CV scans between 0.05 and 1.2 V at a scan rate of 100 mV/s to obtain the initial rapid Cu dissolution profiles. Then, the catalyst films were further pretreated using 200 CV scans between 0.05 and 1.2 V at a scan rate of 500 mV/s during which a large amount of Cu was lost from the alloy nanoparticle precursors. Thereafter, the platinum electrochemical surface area (Pt-ECSA) of each catalyst was determined by cycling the pretreated catalysts at 100 mV/s between 0.05 and 1.0 V. The Pt-ECSA of each catalyst was determined using the mean integral charge of the hydrogen adsorption and desorption areas with the double-layer current corrected for, using
atmosphere. The voltammetric response of the electrocatalysts was first measured during the initial three CV scans between 0.05 and 1.2 V at a scan rate of 100 mV/s to obtain the initial rapid Cu dissolution profiles. Then, the catalyst films were further pretreated using 200 CV scans between 0.05 and 1.2 V at a scan rate of 500 mV/s during which a large amount of Cu was lost from the alloy nanoparticle precursors. Thereafter, the platinum electrochemical surface area (Pt-ECSA) of each catalyst was determined by cycling the pretreated catalysts at 100 mV/s between 0.05 and 1.0 V. The Pt-ECSA of each catalyst was determined using the mean integral charge of the hydrogen adsorption and desorption areas with the double-layer current corrected for, using  , assuming 1 H atom is adsorbed to 1 Pt atom. Linear sweep voltammetry (LSV) measurements were conducted in oxygen-saturated electrolyte by sweeping the potential from 0.06 V anodically to the open-circuit potential
, assuming 1 H atom is adsorbed to 1 Pt atom. Linear sweep voltammetry (LSV) measurements were conducted in oxygen-saturated electrolyte by sweeping the potential from 0.06 V anodically to the open-circuit potential  at a scan rate of 5 mV/s. The ORR activities of the dealloyed, activated catalysts were corrected for mass transport limitation using Eq. 5 in Ref. 22. This equation is the simplest mass transport correction and does not explicitly include a check of whether the
at a scan rate of 5 mV/s. The ORR activities of the dealloyed, activated catalysts were corrected for mass transport limitation using Eq. 5 in Ref. 22. This equation is the simplest mass transport correction and does not explicitly include a check of whether the  -intercept of the Koutecky–Levich plot (
-intercept of the Koutecky–Levich plot ( vs
vs  ) vanishes at high enough electrode potentials. Mass activities
) vanishes at high enough electrode potentials. Mass activities  and specific activities
and specific activities  were then established at 900 mV at room temperature using the relation. The carbon-supported 30 wt% platinum electrocatalyst used as a synthesis precursor is also used as activity and ECSA baseline in the aging experiments.
were then established at 900 mV at room temperature using the relation. The carbon-supported 30 wt% platinum electrocatalyst used as a synthesis precursor is also used as activity and ECSA baseline in the aging experiments.
Powder stability and aging studies
The stability and aging of the carbon-supported  (800/7) alloy nanoparticle catalyst precursor under ambient conditions and in air were studied by weighing four 5 mg samples of the material into four 20 mL scintillation vials. Filter disks were placed over the mouths of the vials and were kept in place using open-top screw caps. Filter disks were used to facilitate air exchange while preventing contamination from dusts or other solid impurities. All four samples were kept in a well-vented part of a clean room for the entire experimental duration. The first vial of sample was used for electrochemical characterization immediately without being aged in air (air aged for 0 days in Fig. 3). The other three vials of the sample were consecutively used for electrochemical characterization after being exposed to air for 5, 12, and 26 days (air aged for 5, 12, and 26 days, respectively). Each powder sample was made into a catalyst ink and immediately dispensed onto the GC surface of an RDE before being analyzed electrochemically. The vials of ink were then returned to the aging storage location for use in the thin-film and ink stability experiments.
(800/7) alloy nanoparticle catalyst precursor under ambient conditions and in air were studied by weighing four 5 mg samples of the material into four 20 mL scintillation vials. Filter disks were placed over the mouths of the vials and were kept in place using open-top screw caps. Filter disks were used to facilitate air exchange while preventing contamination from dusts or other solid impurities. All four samples were kept in a well-vented part of a clean room for the entire experimental duration. The first vial of sample was used for electrochemical characterization immediately without being aged in air (air aged for 0 days in Fig. 3). The other three vials of the sample were consecutively used for electrochemical characterization after being exposed to air for 5, 12, and 26 days (air aged for 5, 12, and 26 days, respectively). Each powder sample was made into a catalyst ink and immediately dispensed onto the GC surface of an RDE before being analyzed electrochemically. The vials of ink were then returned to the aging storage location for use in the thin-film and ink stability experiments.
Figure 3. Effect of aging in air of the dry carbon-supported Pt–Cu alloy nanoparticle precursor on its ECSA and oxygen reduction reactivity: (a) Pt mass activity vs powder aging, (b) Pt surface area (specific) activity vs powder aging, and (c) Pt-based electrochemically active surface area (Pt-ECSA) vs powder aging. Values of the carbon-supported alloy precursor  (circle) are compared to a standard pure Pt/C nanoparticle catalyst (square).
(circle) are compared to a standard pure Pt/C nanoparticle catalyst (square).
Thin-film stability and aging studies
Each of the four ink samples used for the powder stability experiments (Power Stability and Aging Studies section) was returned to their aging location after film dispensing. The following day, the ink was sonicated in a water bath for 15 min at room temperature before being dispensed and cast into a thin film onto the GC surfaces of five different RDE shafts. The five RDE thin films were dried and kept in the aging location. The first thin film was analyzed electrochemically immediately upon drying (age of film: day 2 in Fig. 4), while the other four RDEs were analyzed consecutively for the next four days (age of film: days 3–6 in Fig. 4).
Figure 4. Effect of aging of Pt–Cu alloy nanoparticle precursor catalyst films on electrocatalytic performance: (a) Pt mass activity at 0.9 V/RHE, (b) Pt specific activity at 0.9 V/RHE, (c) surface area (Pt-ECSA) vs film age: 0 days (circle), 5 days (triangle), 12 days (diamond), and 26 days (left triangle), compared with pure Pt/C (square). Catalysts used are carbon-supported  nanoparticles.
nanoparticles.
Catalyst precursor ink stability and aging studies
Each catalyst ink sample was electrochemically characterized for a total of six consecutive days, with the first day being when the powder sample was made into an ink. After the first day (age of ink: day 1) and for the next five days (age of ink: day 2–6), each ink sample was sonicated in a water bath for 15 min at room temperature immediately before being dispensed and cast into a thin film on the GC surface of an RDE. All dried thin films were immediately tested electrochemically to study the stability of the catalyst ink solution. Experimental measurement errors were evaluated in occasional repeat experiments. A relative experimental error of 5–10% in the ECSA values was determined in repeat experiments of the ink stability behavior.
Results and Discussion
Stability and aging of the Pt–Cu powder
In studying the aging effects on the  (800/7) catalyst precursor material, four freshly prepared, weighted powder samples were taken out of their
(800/7) catalyst precursor material, four freshly prepared, weighted powder samples were taken out of their  storage box and exposed to a controlled ambient pressure (0.2 atm oxygen partial pressure), an ambient temperature of about 25°C, and for 0, 5, 12, and 26 days from the time of synthesis. The powders were exposed to ambient conditions such that no solid contamination (dust particles, etc.) were able to deposit onto the powder sample. These precursor powder samples are thereof referred to as "Pt–Cu–
storage box and exposed to a controlled ambient pressure (0.2 atm oxygen partial pressure), an ambient temperature of about 25°C, and for 0, 5, 12, and 26 days from the time of synthesis. The powders were exposed to ambient conditions such that no solid contamination (dust particles, etc.) were able to deposit onto the powder sample. These precursor powder samples are thereof referred to as "Pt–Cu– days–P," where
days–P," where  , 5, 12, 26. After the controlled aging experiment, each powder sample was used to prepare a catalyst ink, which was immediately dispensed onto the GC disk of an RDE. Each thin-film catalyst went through an identical electrochemical pretreatment (dealloying) protocol, and the resulting Pt-enriched Pt–Cu/C thin-film catalyst was analyzed for its ECSA and its electrochemical reactivity for the ORR.
, 5, 12, 26. After the controlled aging experiment, each powder sample was used to prepare a catalyst ink, which was immediately dispensed onto the GC disk of an RDE. Each thin-film catalyst went through an identical electrochemical pretreatment (dealloying) protocol, and the resulting Pt-enriched Pt–Cu/C thin-film catalyst was analyzed for its ECSA and its electrochemical reactivity for the ORR.
Figure 1 shows the final CV profiles of the four aged powder samples (dashed lines) and compares them with that of a standard pure Pt/C electrocatalyst, which had been stored under  . The CV profiles of all Pt–Cu catalysts resemble one another but exhibit a significantly smaller under-potentially deposited hydrogen (
. The CV profiles of all Pt–Cu catalysts resemble one another but exhibit a significantly smaller under-potentially deposited hydrogen ( ) profile than Pt/C between 0.05 and 0.3 V, consistent with the previous work.23, 24, 30, 41 The smaller
) profile than Pt/C between 0.05 and 0.3 V, consistent with the previous work.23, 24, 30, 41 The smaller  profile is probably due to the larger Pt–Cu particle sizes after the annealing process. In addition, the shapes of the
profile is probably due to the larger Pt–Cu particle sizes after the annealing process. In addition, the shapes of the  profiles of Pt–Cu/C are similar to that of Pt/C even though the relative heights of the
profiles of Pt–Cu/C are similar to that of Pt/C even though the relative heights of the  peaks are slightly altered. The shapes of the
peaks are slightly altered. The shapes of the  regions of all air-aged Pt–Cu/C samples are also identical despite the different exposure durations to air. This indicates that the contact with air does not alter the relative affinity of the different Pt facets present on the surface of the nanoparticles to hydrogen adsorption. The massive Cu dealloying process during the activation of the catalysts is likely to offset any differences in Cu oxide formation during the aging.
regions of all air-aged Pt–Cu/C samples are also identical despite the different exposure durations to air. This indicates that the contact with air does not alter the relative affinity of the different Pt facets present on the surface of the nanoparticles to hydrogen adsorption. The massive Cu dealloying process during the activation of the catalysts is likely to offset any differences in Cu oxide formation during the aging.
Figure 1. Cyclic voltammetric profiles of carbon-supported  alloy catalyst precursor powders aged for different periods of time in air under ambient conditions compared to a pure Pt/C state-of-the-art electrocatalyst: 0 days (dashed), 5 days (dotted), 12 days (dashed–dotted), and 26 days (dashed–dotted–dotted), compared with Pt/C (solid). 0.1 M
alloy catalyst precursor powders aged for different periods of time in air under ambient conditions compared to a pure Pt/C state-of-the-art electrocatalyst: 0 days (dashed), 5 days (dotted), 12 days (dashed–dotted), and 26 days (dashed–dotted–dotted), compared with Pt/C (solid). 0.1 M  , 25°C.
, 25°C.
Figure 2 compares the LSV profiles and the Tafel plots of the Pt–Cu/C and Pt/C catalysts. All Pt–Cu/C catalysts show a relatively well-defined flat plateau in the region of the diffusion-limiting current between 0.1 and 0.7 V. The value of the transport-limited current is consistent with earlier studies.32, 33, 39–41, 47 Onset potentials from a mixed kinetic-diffusion control region to a diffusion-limiting region occurred at around 0.7 V. All Pt–Cu/C catalysts exhibited a significant shift in the mixed region toward more positive potentials compared to the pure Pt/C. The potential shift is about  and is again consistent with the delayed onset of oxide formation observed from the CVs during the anodic scan. Tafel plots (inset of Fig. 2) indicate significant intrinsic activity improvements of all Pt–Cu/C catalysts regardless of the duration of their exposure to air. However, the significant differences in the Tafel slopes at high current densities (hcd's) suggest the influence of mass transport effects reflected in the diffusion-limited regions of the air-aged Pt–Cu/C samples.
and is again consistent with the delayed onset of oxide formation observed from the CVs during the anodic scan. Tafel plots (inset of Fig. 2) indicate significant intrinsic activity improvements of all Pt–Cu/C catalysts regardless of the duration of their exposure to air. However, the significant differences in the Tafel slopes at high current densities (hcd's) suggest the influence of mass transport effects reflected in the diffusion-limited regions of the air-aged Pt–Cu/C samples.
Figure 2. LSV displaying the oxygen reduction performance of carbon-supported  powders aged for different periods of time in air compared to a fresh pure Pt state-of-the-art electrocatalyst: 0 days (circle), 5 days (triangle), 12 days (inverted triangle), and 26 days (diamond), compared with Pt/C (square). Inset: Tafel plots of electrode potentials vs Pt real surface area (Pt ECSA) normalized (specific) oxygen reduction current density. 0.1 M
powders aged for different periods of time in air compared to a fresh pure Pt state-of-the-art electrocatalyst: 0 days (circle), 5 days (triangle), 12 days (inverted triangle), and 26 days (diamond), compared with Pt/C (square). Inset: Tafel plots of electrode potentials vs Pt real surface area (Pt ECSA) normalized (specific) oxygen reduction current density. 0.1 M  , 25°C, 5 mV/s, anodic direction.
, 25°C, 5 mV/s, anodic direction.
The Tafel slopes at the low current density (lcd) region are close to the theoretical Tafel slopes of 60 mV/dec and that observed from bulk and single crystals reported previously.22–24, 35 In the hcd region, the Tafel slopes are slightly larger than those obtained from the Pt reference samples. The reason for the change in the Tafel slopes between lcd and hcd had been in much discussion. Gasteiger et al. and Paulus et al. explained that the changes in the Tafel slope are due to the changes in the oxide species adsorption on the catalyst surface and to an increase in inaccuracy in the RDE mass transport corrections near the diffusion-limited current density, respectively.22, 23
The Pt mass-based transport-corrected kinetic current density at 0.9 V (Pt mass-based activity)  , the real surface area normalized current density at 0.9 V (specific activity)
, the real surface area normalized current density at 0.9 V (specific activity)  , and the ECSA of the four aged powder samples and the pure Pt/C sample are compared in Fig. 3. From the Pt-ECSA comparison plot (Fig. 3c), it is seen that Pt-ECSA increases as the duration of exposure to air increases, until the 12th day, and then decreases from the 12th to the 26th day. This observation can be attributed to a suboptimal interaction between the Nafion solution and the metal particles and carbon support pores (together controlling the catalyst utilization) of the Pt–Cu–0 day–P, leading to nonoptimized three-phase boundaries on the surface of the Pt–Cu nanoparticles. This, in turn, leads to some Pt–Cu nanoparticles not being in contact with the acidic electrolyte and oxygen, thus not participating in the ORR, lowering the observed catalytic activities.48, 49
, and the ECSA of the four aged powder samples and the pure Pt/C sample are compared in Fig. 3. From the Pt-ECSA comparison plot (Fig. 3c), it is seen that Pt-ECSA increases as the duration of exposure to air increases, until the 12th day, and then decreases from the 12th to the 26th day. This observation can be attributed to a suboptimal interaction between the Nafion solution and the metal particles and carbon support pores (together controlling the catalyst utilization) of the Pt–Cu–0 day–P, leading to nonoptimized three-phase boundaries on the surface of the Pt–Cu nanoparticles. This, in turn, leads to some Pt–Cu nanoparticles not being in contact with the acidic electrolyte and oxygen, thus not participating in the ORR, lowering the observed catalytic activities.48, 49
Both the  and
and  plots (Fig. 3a and 3b) show that all Pt–Cu/C samples exhibit significantly higher catalytic activities than Pt/C, with the highest
plots (Fig. 3a and 3b) show that all Pt–Cu/C samples exhibit significantly higher catalytic activities than Pt/C, with the highest  being
being  and
and  being
being  of the Pt–Cu–5 days–P material. These ORR activity values lie clearly above the technological target performance of the fuel cell cathode catalysts as set out by the Department of Energy for 2010 50 and are consistent with the high ORR activity reported earlier.30, 42, 43 The activities increased from Pt–Cu–0 days–P to Pt–Cu–5 days–P and remained about constant for Pt–Cu–12 days–P and Pt–Cu–26 days–P. Referring to Fig. 3a, it is not surprising that
of the Pt–Cu–5 days–P material. These ORR activity values lie clearly above the technological target performance of the fuel cell cathode catalysts as set out by the Department of Energy for 2010 50 and are consistent with the high ORR activity reported earlier.30, 42, 43 The activities increased from Pt–Cu–0 days–P to Pt–Cu–5 days–P and remained about constant for Pt–Cu–12 days–P and Pt–Cu–26 days–P. Referring to Fig. 3a, it is not surprising that  improved as Pt-ECSA increases because more Pt surface is available for ORR. In addition,
improved as Pt-ECSA increases because more Pt surface is available for ORR. In addition,  (Fig. 3b) is observed to also follow the same trend. This is so despite an increase in Pt-ECSA for the samples air-aged from 0 to 12 days. However, when Pt–Cu nanoparticles were exposed to air for more than 12 days, a drop in both Pt-ECSA and absolute catalytic activities was observed. Because each of the powder samples originated from a single Pt–Cu/C parent source, any changes in electrochemical characteristics must be due to the effects of the oxidation of the nanoparticles in air. The Pt–Cu/C powder samples were thus stable in air for at least 12 days without adversely affecting their electrochemical properties.
(Fig. 3b) is observed to also follow the same trend. This is so despite an increase in Pt-ECSA for the samples air-aged from 0 to 12 days. However, when Pt–Cu nanoparticles were exposed to air for more than 12 days, a drop in both Pt-ECSA and absolute catalytic activities was observed. Because each of the powder samples originated from a single Pt–Cu/C parent source, any changes in electrochemical characteristics must be due to the effects of the oxidation of the nanoparticles in air. The Pt–Cu/C powder samples were thus stable in air for at least 12 days without adversely affecting their electrochemical properties.
Stability and aging of the Pt–Cu thin films
Figure 4 shows the Pt mass-based ORR activity at 0.9 V  , the specific activity at 0.9 V
, the specific activity at 0.9 V  , and the Pt-ECSA of aged Pt–Cu/C catalyst thin films prepared from the four Pt–Cu/C powder samples described in the previous section. The nomenclature for the catalyst thin films used hereforth follows "Pt–Cu–
, and the Pt-ECSA of aged Pt–Cu/C catalyst thin films prepared from the four Pt–Cu/C powder samples described in the previous section. The nomenclature for the catalyst thin films used hereforth follows "Pt–Cu– days films" with
days films" with  denoting the number of days the precursor powder was exposed to air; i.e., "Pt–Cu–26 days film" would refer to a film made from the powder "Pt–Cu–26 days–P." Within seconds, a set of five ink-cast, dry films was prepared on five identical commercial GC electrodes from each aged precursor powder. The time elapsed between the preparation of the liquid catalyst ink and the casting of the catalyst thin film was on the order of a few hours. After their preparation, the individual catalyst thin films were aged under identical ambient conditions from up to 6 days, and one thin film each was catalytically tested on consecutive days.
denoting the number of days the precursor powder was exposed to air; i.e., "Pt–Cu–26 days film" would refer to a film made from the powder "Pt–Cu–26 days–P." Within seconds, a set of five ink-cast, dry films was prepared on five identical commercial GC electrodes from each aged precursor powder. The time elapsed between the preparation of the liquid catalyst ink and the casting of the catalyst thin film was on the order of a few hours. After their preparation, the individual catalyst thin films were aged under identical ambient conditions from up to 6 days, and one thin film each was catalytically tested on consecutive days.
From the Pt-ECSA plot of Fig. 4c, the active Pt surface area of the aged films generally decreases with time, evidencing a significant impact of dry age and air exposure on the precursor films. The significant drop in Pt-ECSA of the films ( to 50%) suggests that either interactions between air and surfaces of Nafion embedded Pt–Cu alloy nanoparticles or the irreversible aging processes, such as cracking, over the prolonged drying duration had altered the mesoscopic and microscopic film network structure such that the achievable
to 50%) suggests that either interactions between air and surfaces of Nafion embedded Pt–Cu alloy nanoparticles or the irreversible aging processes, such as cracking, over the prolonged drying duration had altered the mesoscopic and microscopic film network structure such that the achievable  charge after identical dealloying of the Pt–Cu nanoparticles decreased. We hypothesize that the continued film cracking would impact the structure and functionality of the proton-conducting network, which is critical in the discharge of protons at the catalytically active surface.
charge after identical dealloying of the Pt–Cu nanoparticles decreased. We hypothesize that the continued film cracking would impact the structure and functionality of the proton-conducting network, which is critical in the discharge of protons at the catalytically active surface.
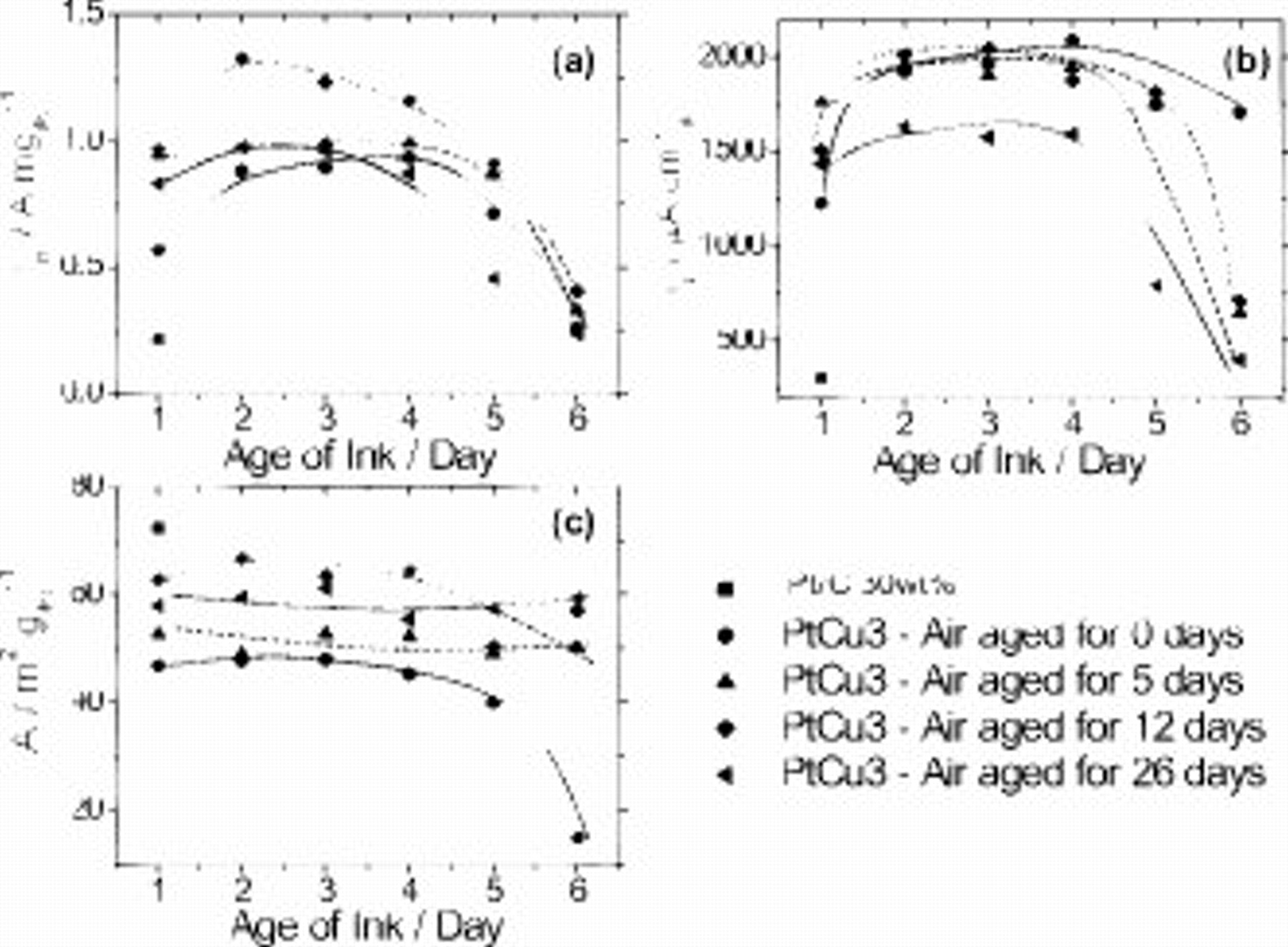
In terms of the ORR activities as a function of film age, there is an overall downward trend in both  and
and  for all four sets of five thin films each. The activity values,
for all four sets of five thin films each. The activity values,  and
and  , of all samples were significantly above that of our standard Pt/C at the beginning, yet they fell rapidly and approached the initial activity values of pure Pt/C by day 4.
, of all samples were significantly above that of our standard Pt/C at the beginning, yet they fell rapidly and approached the initial activity values of pure Pt/C by day 4.
An intraday ORR activity comparison between films from different powders (Fig. 4a and 4b) reveals a monotonously decreasing ORR activity with age, with the "Pt–Cu–12 days–film" exhibiting the highest activity among the different powders. This is in line with the measurement of Fig. 3 where that powder sample ranged among the most active when measured in the form of fresh ink samples. Surprisingly, the absolute values of the mass and specific activities are higher on 2-days old thin films compared to fresh ones ("day 1" in Fig. 3). In view of the combined drop of ECSA in Fig. 4c, this observation is even more surprising. On subsequent days, the experimental mass and specific activities fell rapidly and converged at the end of the experiment. The observed activity trajectories are consistent with the hypothesis that Pt-ECSA decreases with time due to degradation and cracking of the Nafion film network: The number of active precursor Pt–Cu nanoparticles participating in the dealloying and ORR catalysis gradually reduced, causing a drop in activities.
To study and understand the deterioration of the Pt–Cu/C films, the Tafel plots of "Pt–Cu–12 days–films" and "Pt–Cu–26–days films" are presented in Fig. 5 for all days of aging. The two sets of films were chosen because they represented the highest and lowest performing catalytic films over the duration of the experiment, respectively, not taking into account the "Pt–Cu–0 days–films." Figure 5a evidences that the lcd and hcd Tafel slopes of the set of films from the 12 day old power were essentially parallel to those of the initial Pt/C Tafel slopes. However, in Fig. 5b, it is observed that even though the Tafel slopes of this set of films at lcd were essentially parallel to Pt/C, those at hcd were much steeper than that of Pt/C. This increase in the Tafel slope typically indicates mass diffusion transport limitations of protons, water, or reactant molecules possibly caused by changes in the mesoscopic film network structure due to the prolonged drying of the catalyst film.35 So, the detrimental processes of air and prolonged drying rendered the precursor Pt–Cu/C thin films unsuitable for the ORR activity measurement even after just a day.
Figure 5. Tafel plots of aged precursor catalyst films derived from measurements from Fig. 4. (a) Data obtained from powder air aged for 12 days and (b) data obtained from powder air aged for 26 days: Day 2 (triangle), day 3 (inverted triangle), day 4 (diamond), and day 5 (left-pointing triangle), compared with pure Pt/C (square). Catalysts used are carbon-supported  nanoparticles.
nanoparticles.
Stability and aging of the Pt–Cu inks
We also investigated the effect of aging and air exposure to liquid precursor powder inks, that is, liquid suspensions of the precursor powder mixed with a Nafion ionomer. As before, the Pt–Cu/C precursor inks were dispensed as thin films on a rotating GC electrode and immediately tested for ECSA and electrochemical ORR activity. Thereafter, the tested ink batch was returned to the controlled aging place and left there. This study covered an aging range of up to 6 days. The nomenclature of the inks used from hereon is "Pt–Cu– days ink," with
days ink," with  number of days the original powder was left aging in ambient conditions. Figure 6 shows the experimental
number of days the original powder was left aging in ambient conditions. Figure 6 shows the experimental  ,
,  , and Pt-ECSA values as the function of age of the four sets of inks made from the same four aged precursor powders.
, and Pt-ECSA values as the function of age of the four sets of inks made from the same four aged precursor powders.
Figure 6. Effects of aging of Pt–Cu alloy nanoparticle precursor catalyst inks on electrochemical performance: (a) Pt mass activity at 0.9 V/RHE, (b) Pt specific activity at 0.9 V/RHE, and (c) surface area (Pt-ECSA): 0 days (circle), 5 days (triangle), 12 days (diamond), and 26 days (left triangle), compared with pure Pt/C (square). Catalysts used are carbon-supported  nanoparticles.
nanoparticles.
From the Pt-ECSA plot (Fig. 6c), it is observed that with the exception of the day 6 surface area of the ink made from the fresh precursor powder, the ECSA of each ink does not vary significantly with time. An intraday comparison of the Pt-ECSA values again reveals a maximum for the 12-days old powder and its inks. The liquid precursor ink is apparently a suitable format in which the favorable ECSA characteristics of the dry powder can be preserved for over 5–6 days. As seen above, dry films do not provide this capability. We hypothesize that the increase in Pt-ECSA suggests many participating Pt surface atoms in the hydrogen underpotential deposition process.
Looking closer at the ECSA values as a function of time, a moderate increase in the second day of measurement of the ink is observed for most powders. The trends in Fig. 6c were found reproducible in repeat experiments with experimental surface area values showing a 5–10% relative error. We hypothesize that the wetting of micropores and, hence, slightly enlarging the contact surface between the Nafion ionomer, metal particles and carbon support over the first 24 h of ink aging51 and that this could result in a moderate increase in ECSA. However, the ECSA increase is unlikely to be responsible for the evolution of the ORR activity of the aging ink because the catalytic ORR activities of the ink samples were as high as four to six times that of the standard pure Pt/C by day 2 and then converged back to that of pure Pt/C by day 6. Importantly, a significant increase in  and
and  activities was observed for most inks after 1 day of aging; the activities then remained essentially constant before they dropped on days 5 and 6. The
activities was observed for most inks after 1 day of aging; the activities then remained essentially constant before they dropped on days 5 and 6. The  activity of the ink made from the 12-days old powder exhibited a particularly significant activity boost after the first day ("day 2" in Fig. 6) but then gradually converged with the performances of other inks over the period of the experiment. Clearly, the moderate increase in the ECSA value on day 2 cannot be responsible for the significant ORR activity increases. There appear to be other activity controlling characteristics than merely the number of electrochemically accessible surface Pt atoms, which manipulate the observed ORR activity. We hypothesize that the improved three-phase interface between metal, carbon pores, and ionomer on day 2 may create more favorable conditions for the dealloying of the precursor material. The prolonged storage of the Pt–Cu alloy precursor in the ionomer ink may cause a premature molecular oxygen-induced chemical leaching of the Cu surface atoms, which maintains a fresh unoxidized metal surface and may result in a more complete leaching of the precursor during the electrochemical process.40, 45
activity of the ink made from the 12-days old powder exhibited a particularly significant activity boost after the first day ("day 2" in Fig. 6) but then gradually converged with the performances of other inks over the period of the experiment. Clearly, the moderate increase in the ECSA value on day 2 cannot be responsible for the significant ORR activity increases. There appear to be other activity controlling characteristics than merely the number of electrochemically accessible surface Pt atoms, which manipulate the observed ORR activity. We hypothesize that the improved three-phase interface between metal, carbon pores, and ionomer on day 2 may create more favorable conditions for the dealloying of the precursor material. The prolonged storage of the Pt–Cu alloy precursor in the ionomer ink may cause a premature molecular oxygen-induced chemical leaching of the Cu surface atoms, which maintains a fresh unoxidized metal surface and may result in a more complete leaching of the precursor during the electrochemical process.40, 45
The observed  value of about
value of about  for the 12 day old powder on the second day of the ink represents one of the highest Pt mass activities ever reported for nanoparticle alloy electrocatalysts. This finding is likely to be of great importance for the development of practical ink preparation and membrane electrode assembly (MEA) building recipes.
for the 12 day old powder on the second day of the ink represents one of the highest Pt mass activities ever reported for nanoparticle alloy electrocatalysts. This finding is likely to be of great importance for the development of practical ink preparation and membrane electrode assembly (MEA) building recipes.
The Tafel plots of inks made from Pt–Cu–0 days–P and Pt–Cu–12 days–P, tested for 6 consecutive days, are shown in Fig. 7. As with Fig. 5, the lcd region of the Tafel plots of all Pt–Cu/C samples were parallel to Pt/C but shifted toward an hcd expressing the favorable ORR activity of dealloyed Pt–Cu electrocatalysts. The evolution of the Tafel slopes of the two sets of inks over time is consistent with our previous observations and conclusions and sheds light on the evolution of the activity with ink age. The day 1 and day 6 ink made from the "Pt–Cu–0 days–P" powder deviated clearly from the Tafel slope of Pt/C at hcd. The inks aged for durations between 2–5 days were fairly parallel to the standard Pt/C. The same can be said of the ink sample prepared from Pt–Cu–12 days–P.
Figure 7. Tafel plots of aged precursor catalyst inks derived from measurements from Fig. 6. (a) Data obtained from powder air aged for 0 days and (b) data obtained from powder air aged for 12 days: Day 1 (circle), day 2 (triangle), day 3 (inverted triangle), day 4 (diamond), and day 5 (left-pointing triangle), compared with pure Pt/C (square). Catalysts used are carbon-supported  nanoparticles.
nanoparticles.
This observation again suggests that catalyst films made from fresh and very old inks exhibit serious diffusion limitations and lower activities. Our studies suggest that the inks appear to require some moderate aging of about 24–48 h after preparation to unfold their full activity. This finding has important implications for the development of optimized ink preparation recipes for fuel cell catalyst inks and pastes. Again, prolonged aging beyond the optimum resulted in a poor film quality and severe diffusion limitations.
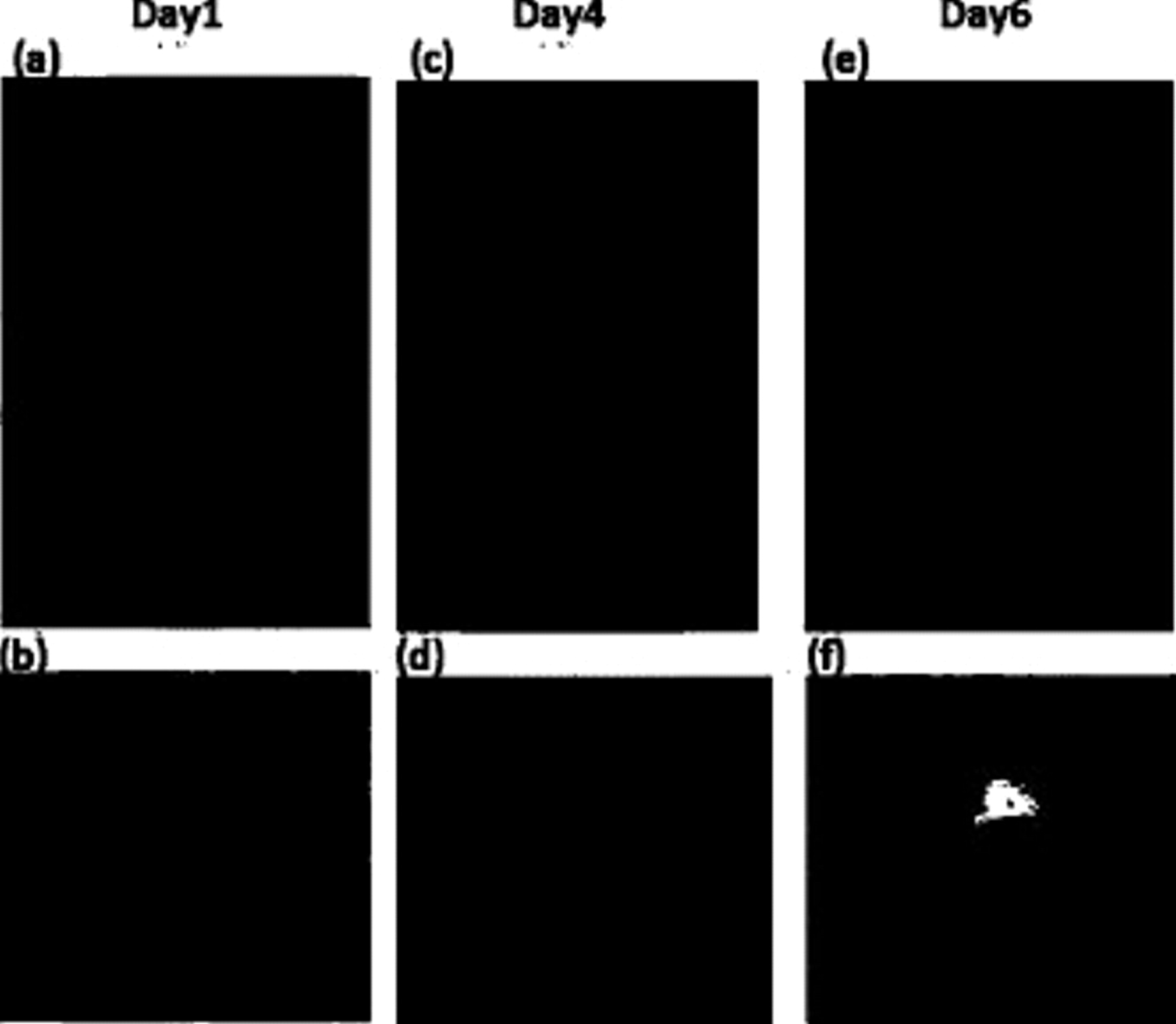
Our conclusions about the existence of an optimal ink age are corroborated by visual observations of the stability of the ink suspension. A suspension of poor stability appears clear with precursor powder settling to the bottom of the vial, as shown in Fig. 8. Figure 8a, 8c and 8e shows a "Pt–Cu–26 days–ink" on day 1 when the ink was freshly prepared, day 4, and day 6, respectively. Figure 8b, 8d and 8f reports a micrograph of the corresponding catalyst thin films. On day 1, the freshly prepared ink suspension was stable, evidenced by its uniformly black appearance. The carbon-supported catalyst particles remained suspended even in the absence of external stirring or sonication. The corresponding catalyst thin film was spatially very uniform in thickness across the 5 mm diameter GC electrode (Fig. 8b). The visual quality of the ink and film then remained unchanged over the following 2 days where the ink/film reached their optimum performance. As the aqueous Nafion catalyst ink aged further, the visual appearance of the catalyst ink changed in that small black carbon/catalyst agglomerates formed and stick to the wall of the vial. The ink therefore started to clear up. At the same time, the ink showed increased hydrophobicity evidenced by its increasing affinity to deposit and bind to the hydrophobic Teflon shaft of the RDE, in which the GC electrode surface was embedded. Dispensing of the ink onto the GC without forming spontaneous deposits on the Teflon became impossible (Fig. 8d). The film formed upon drying was nonuniform in thickness and electrode coverage. This increase in hydrophobicity suggested a change in the carbon black particle surface properties. Carbon blacks in the dry state are rather hydrophobic in nature; however, ultrasonication aided by polyelectrolytes can cause water molecules to enter the carbon pores and to make it behave rather hydrophilically. The data in Fig. 6 demonstrated that during days 4 and 5, the inks/films began to drop in catalytic ORR activities. By day 6 of the aging experiment, the borosilicate wall of the scintillation vial was fully covered by a thin layer of black catalyst agglomerates. The ink suspension was now almost completely clear. The catalyst thin film formed was nonuniform with large uncovered islands of the exposed GC surface. In Fig. 6c, a poor film quality did not seem to affect the Pt-ECSA of the samples; however, the nonuniform film exhibited very low  and
and  . In addition, Tafel slopes of the 6 day old ink exhibited significant deviations from the standard values indicated suboptimal mass transport.
. In addition, Tafel slopes of the 6 day old ink exhibited significant deviations from the standard values indicated suboptimal mass transport.
Figure 8. Visual effects of aging of a catalyst precursor ink and its corresponding RDE catalyst thin film. The ink used was a "Pt–Cu–26 days–ink" that is prepared from a powder, which was aged for 26 days under ambient conditions in air. (a) Fresh ink, vial wall is clean; (b) film from fresh ink, uniform film only covering the GC electrode; (c) 4 day old ink, particles stuck to wall of vial; (d) film from a 4 day old ink, film uniformity deteriorates, film sticks to Teflon shaft (white) of RDE; (e) 6 day old ink, carbon/catalyst particles conglomerate and separate from suspension, particles stick to wall of vial; and (f) film from a 6 day old ink, a very poor film coverage on the GC surface.
Conclusions
Most electrochemical researchers today use the RDE method to test the electrocatalytic performance of carbon-supported alloy nanoparticles. The RDE method involves the preparation of catalyst inks and the casting of catalyst thin films. The observed performance of the catalyst depends critically on the properties of ink and film. In most such studies to date, the effect of aging and handling of the catalyst powder, its ink, and thin film on the measured catalytic characteristics has not been addressed. To the best of our knowledge, this is the systematic study that considers the effect of aging on the electrocatalytic properties of carbon-supported Pt alloy nanoparticle electrocatalysts in three different formats of powder, liquid ink, and dried thin films. In particular, we have addressed the aging behavior of carbon-supported  nanoparticle alloy catalyst precursor materials. These precursors are used for the preparation of the highly active ORR fuel cell electrocatalyst by selective chemical or voltammetric dissolution (dealloying) of Cu surface atoms from the alloy particles. The dealloying process results in a core-shell catalyst with the unprecedented Pt mass and specific ORR activity. The high molar content of Cu in the precursor raised concerns about the stability of the precursor in ambient conditions and oxygen.
nanoparticle alloy catalyst precursor materials. These precursors are used for the preparation of the highly active ORR fuel cell electrocatalyst by selective chemical or voltammetric dissolution (dealloying) of Cu surface atoms from the alloy particles. The dealloying process results in a core-shell catalyst with the unprecedented Pt mass and specific ORR activity. The high molar content of Cu in the precursor raised concerns about the stability of the precursor in ambient conditions and oxygen.
Our investigations allow three major conclusions about the aging behavior of the considered alloy nanoparticles:
- (1)The aging of dry carbon-supported
 nanoparticles in ambient conditions does not significantly affect the performance of the dealloyed electrocatalysts. Dry aging led to a moderate performance maximum after about 12 days, yet converges back to initial catalytic ORR performance after about a month of exposure and aging. This indicates that the sensitivity of the catalyst precursor powders to ambient conditions is limited and suggests that no extraordinary measures to protect the precursor from air are necessary.
nanoparticles in ambient conditions does not significantly affect the performance of the dealloyed electrocatalysts. Dry aging led to a moderate performance maximum after about 12 days, yet converges back to initial catalytic ORR performance after about a month of exposure and aging. This indicates that the sensitivity of the catalyst precursor powders to ambient conditions is limited and suggests that no extraordinary measures to protect the precursor from air are necessary. - (2)Freshly dried catalyst thin films mirror the relative activity trends of aged powders. Aging films, however, show a severe monotonous decrease in performance with aging time on the order of days. Catalyst precursor films appear to be the most sensitive format to aging and require special care and handling. Our study was not designed to separate between the effect of molecular oxygen and age. We conclude that prolonged film drying leads to mechanical changes in morphology (cracking, etc.), which deteriorate the ionomer network of the three-phase interface and thus affect the kinetic performance.
- (3)Liquid aqueous Nafion inks exhibit an optimum age of about 24–48 h. At that age, their casted films show the highest ORR activities after dealloying and highest ECSA values. However, the ECSA values cannot fully account for the activity increase, which is why improved dealloying conditions are hypothesized with moderate aging. Wetting of the catalyst pores is likely to increase after aging the ink for 1–2 days. This finding is of general importance for the preparation and behavior of catalyst inks and bears critical implications for the design of improved catalyst preparation recipes. The quality of ink can be monitored by visual inspection, as outlined here.
The practical implications from our study about handling and formulating base metal-rich precursor materials are that such powders can be stored in dry form for a longer time without compromising their activities. Catalyst inks that are prepared should be left aging for at least 24 h and up to 48 h before film casting or MEA preparation is performed. Electrochemical measurements using a given ink can be carried out until the fourth day, at which time the catalyst ink should be discarded. All catalyst thin films on RDEs should be prepared just before the actual measurements commence to minimize the degradation of the films.
Our study is unable to provide an atomic scale insight into the complex processes associated with air aging. Much work and sophisticated methods are going to be required to better characterize, understand, and optimize the triple-phase boundary-supported fuel cell catalyst systems.
Acknowledgments
This project was supported by the National Science Foundation (NSF) program "energy for sustainability" under award no. 0729722 . The support by the State of Texas through the Advanced Research Program during the 2008–2010 funding period is gratefully acknowledged. P.S. acknowledges support by the DFG-funded cluster of excellence Unified Concepts in Catalysis (Unicat) at the Technical University Berlin.
Technical University Berlin assisted in meeting the publication costs of this article.