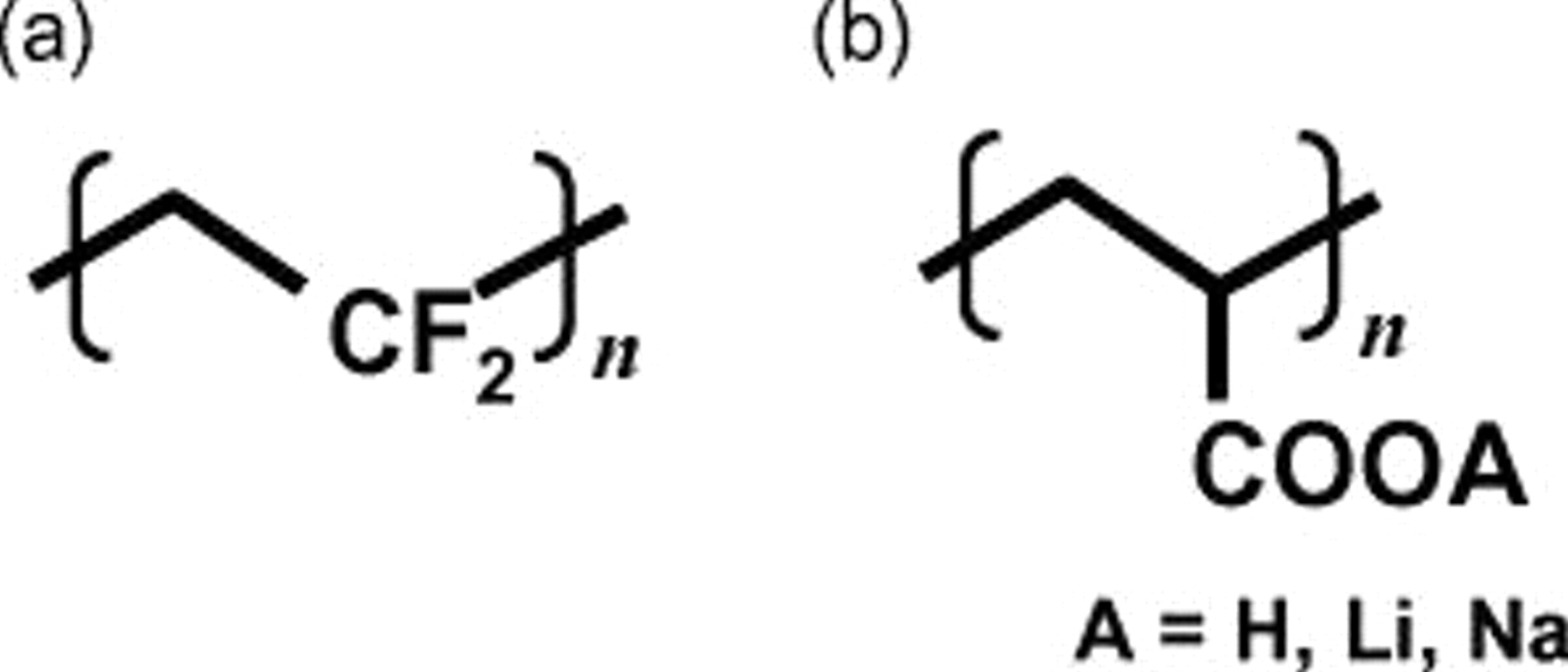Abstract
Polyacrylates were applied as a binder of graphite electrode in a lithium-ion cell to modify the interface. Compared to a conventional binder, poly(vinylidene fluoride), the efficiency at the initial cycle was improved by poly(acrylic acid) and alkali polyacrylates in an ethylene carbonate (EC)-based electrolyte with highly reversible lithium intercalation. In a  propylene carbonate (PC) solution, the poly(vinylidene fluoride) electrode showed a huge irreversible capacity as is generally known. However, the polyacrylate-modified graphite demonstrated highly reversible lithium intercalation in the PC electrolyte containing no film-forming additives as well as in the EC electrolyte due to the interfacial modification with polyacrylates.
propylene carbonate (PC) solution, the poly(vinylidene fluoride) electrode showed a huge irreversible capacity as is generally known. However, the polyacrylate-modified graphite demonstrated highly reversible lithium intercalation in the PC electrolyte containing no film-forming additives as well as in the EC electrolyte due to the interfacial modification with polyacrylates.
Export citation and abstract BibTeX RIS
For the negative electrode of Li-ion cells, graphite exhibits a high specific capacity, low working potential close to that of  couple, and superior cycling behavior. When graphite powders are utilized as the active material, the irreversible capacity appears at the first cycle, because reductive decomposition of the electrolyte solution occurs at the graphite/electrolyte interface during the first charge (electroreduction) including the formation of solid electrolyte interphase (SEI).1, 2 The existence of an SEI layer plays an important role in reversible lithium intercalation into interspace between graphene slabs. The kinetics of lithium intercalation is predominantly determined by the SEI, because all lithium ions in an electrolyte solution must cross the interphase accompanied with desolvation in the case of intercalation; therefore, the chemical modification of a graphite surface including an SEI layer has attracted wide attention to improve battery performance.
couple, and superior cycling behavior. When graphite powders are utilized as the active material, the irreversible capacity appears at the first cycle, because reductive decomposition of the electrolyte solution occurs at the graphite/electrolyte interface during the first charge (electroreduction) including the formation of solid electrolyte interphase (SEI).1, 2 The existence of an SEI layer plays an important role in reversible lithium intercalation into interspace between graphene slabs. The kinetics of lithium intercalation is predominantly determined by the SEI, because all lithium ions in an electrolyte solution must cross the interphase accompanied with desolvation in the case of intercalation; therefore, the chemical modification of a graphite surface including an SEI layer has attracted wide attention to improve battery performance.
With the aim of enhancing the negative electrode performance in lithium-based rechargeable batteries, various inorganic additives dissolved into an electrolyte solution are known to be effective:  ,3 HF,4
,3 HF,4  , and
, and  5 for metallic lithium and cobalt ion,6 lithium carbonate,7 and sodium ion8 for carbon anodes. These additives successfully suppress the initial irreversibility and improve cycle performance. We previously found several particular additives for
5 for metallic lithium and cobalt ion,6 lithium carbonate,7 and sodium ion8 for carbon anodes. These additives successfully suppress the initial irreversibility and improve cycle performance. We previously found several particular additives for  batteries, vinyl-pyridine,9 LiI, LiBr, and
batteries, vinyl-pyridine,9 LiI, LiBr, and  .10
.10
In previous literatures, the electrochemical lithiation properties of carbon anode were improved by its surface treatment with metal oxides11 and lithium carbonate.12 Our group has recently emphasized the key role of the inorganic ingredient at the graphite surface, such as lithium, sodium, potassium,13 and fluorine,14 and we described the impact of Na salts as an electrolyte additive15 and coating16, 17 on the enhancement of battery performance. Recently, we found that sodium polyacrylate and analogous polymers18–20 are capable of depressing the electrochemically irreversible reaction in 
 propylene carbonate (PC) solution without any film-forming additives at highly crystalline graphite electrode, though the conventional poly(vinylidene fluoride) (PVdF) never showed such an effect. Because PC has a much lower freezing point
propylene carbonate (PC) solution without any film-forming additives at highly crystalline graphite electrode, though the conventional poly(vinylidene fluoride) (PVdF) never showed such an effect. Because PC has a much lower freezing point  than ethylene carbonate (EC) (ca.
than ethylene carbonate (EC) (ca.  ), improved ionic conductivity of PC-based electrolyte at lower temperatures can be achieved for lithium-ion cells. As previously reported, several electrolyte additives were also effective in suppressing the decomposition at graphite electrode in the PC-based electrolytes.21 In this paper, we report the effects of three polyacrylates (Fig. 1), poly(acrylic acid) (PAAH), lithium polyacrylate (PAALi), and sodium poly acrylate (PAANa), as both an interface modifier and a binder, upon the electrochemical lithium intercalation into graphite.
), improved ionic conductivity of PC-based electrolyte at lower temperatures can be achieved for lithium-ion cells. As previously reported, several electrolyte additives were also effective in suppressing the decomposition at graphite electrode in the PC-based electrolytes.21 In this paper, we report the effects of three polyacrylates (Fig. 1), poly(acrylic acid) (PAAH), lithium polyacrylate (PAALi), and sodium poly acrylate (PAANa), as both an interface modifier and a binder, upon the electrochemical lithium intercalation into graphite.
Figure 1. Molecular structure of binders used in this study: (a) conventional PVdF and (b) polyacrylates (PAAH, PAALi, and PAANa for  , Li, and Na, respectively).
, Li, and Na, respectively).
Experimental
Reagent-grade natural graphite (particle size ca.  in diameter, surface area
in diameter, surface area  ), PAAH [molecular weight
), PAAH [molecular weight  , Sigma-Aldrich Co.], and PVdF were used. To prepare lithium and sodium polyacrylates (PAALi and PAANa, respectively), PAAH aqueous solutions were neutralized by adding LiOH and NaOH aqueous solutions, respectively. Battery-grade lithium foil, EC, dimethyl carbonate (DMC), PC, and
, Sigma-Aldrich Co.], and PVdF were used. To prepare lithium and sodium polyacrylates (PAALi and PAANa, respectively), PAAH aqueous solutions were neutralized by adding LiOH and NaOH aqueous solutions, respectively. Battery-grade lithium foil, EC, dimethyl carbonate (DMC), PC, and  were used without any further purification and treatment.
were used without any further purification and treatment.
For preparation of working electrodes, the graphite powders  were mixed with a binder
were mixed with a binder  such as PVdF in
such as PVdF in  -methylpyrrolidinone and PAAH, PAALi, and PAANa in water. The black slurry thus obtained was coated onto nickel mesh. The electrodes were dried at
-methylpyrrolidinone and PAAH, PAALi, and PAANa in water. The black slurry thus obtained was coated onto nickel mesh. The electrodes were dried at  in a vacuum state prior to use. A beaker-type electrochemical cell, which consisted of the graphite working electrode, a lithium foil as a quasi-reference electrode, and electrolyte was assembled in an Ar-filled glove box (dew point
in a vacuum state prior to use. A beaker-type electrochemical cell, which consisted of the graphite working electrode, a lithium foil as a quasi-reference electrode, and electrolyte was assembled in an Ar-filled glove box (dew point  ). The electrolytes used were
). The electrolytes used were 
 in EC:DMC (1:1 by volume) and
in EC:DMC (1:1 by volume) and 
 in PC. Galvanostatic charge and discharge (lithium intercalation and deintercalation, respectively) tests of the graphite as a negative electrode at
in PC. Galvanostatic charge and discharge (lithium intercalation and deintercalation, respectively) tests of the graphite as a negative electrode at  (corresponding to C/7 rate in the case of
(corresponding to C/7 rate in the case of  ) were carried out between 0.0 and
) were carried out between 0.0 and  vs Li at
vs Li at  .
.
Results and Discussion
PVdF is broadly known to be used as a binder to prepare the graphite and  powder electrodes of lithium-ion batteries. There is a general consensus that PVdF is chemically stable and that its binding ability is adequate for the sake of lithium-ion cells, so PVdF is used for testing the electrochemical insertion of lithium into various materials in powder state; additionally, polytetrafluoroethylene, (styrene-butadiene rubber)-(sodium carboxymethyl cellulose), PAAH, etc., 22, 23 are commonly used as electrode binder components. In this investigation, we chose polyacrylates not only to bind graphite powders but also to modify the electrode surface in comparison with the typical binder, PVdF. Figure 1 compares the molecular structures. Polyacrylates are soluble in water, resulting in easy handling and organic solvent free-process of electrode preparation despite few PVdF-soluble organic solvents. Because alkali ions influence the interfacial properties of carbon (graphite) anode as described previously,13, 15–17 one can expect the combined effect of polyacrylates and alkali ions of PAALi and PAANa, which are prepared by neutralizing PAAH with equivalent amounts of LiOH and NaOH solutions, respectively.
powder electrodes of lithium-ion batteries. There is a general consensus that PVdF is chemically stable and that its binding ability is adequate for the sake of lithium-ion cells, so PVdF is used for testing the electrochemical insertion of lithium into various materials in powder state; additionally, polytetrafluoroethylene, (styrene-butadiene rubber)-(sodium carboxymethyl cellulose), PAAH, etc., 22, 23 are commonly used as electrode binder components. In this investigation, we chose polyacrylates not only to bind graphite powders but also to modify the electrode surface in comparison with the typical binder, PVdF. Figure 1 compares the molecular structures. Polyacrylates are soluble in water, resulting in easy handling and organic solvent free-process of electrode preparation despite few PVdF-soluble organic solvents. Because alkali ions influence the interfacial properties of carbon (graphite) anode as described previously,13, 15–17 one can expect the combined effect of polyacrylates and alkali ions of PAALi and PAANa, which are prepared by neutralizing PAAH with equivalent amounts of LiOH and NaOH solutions, respectively.
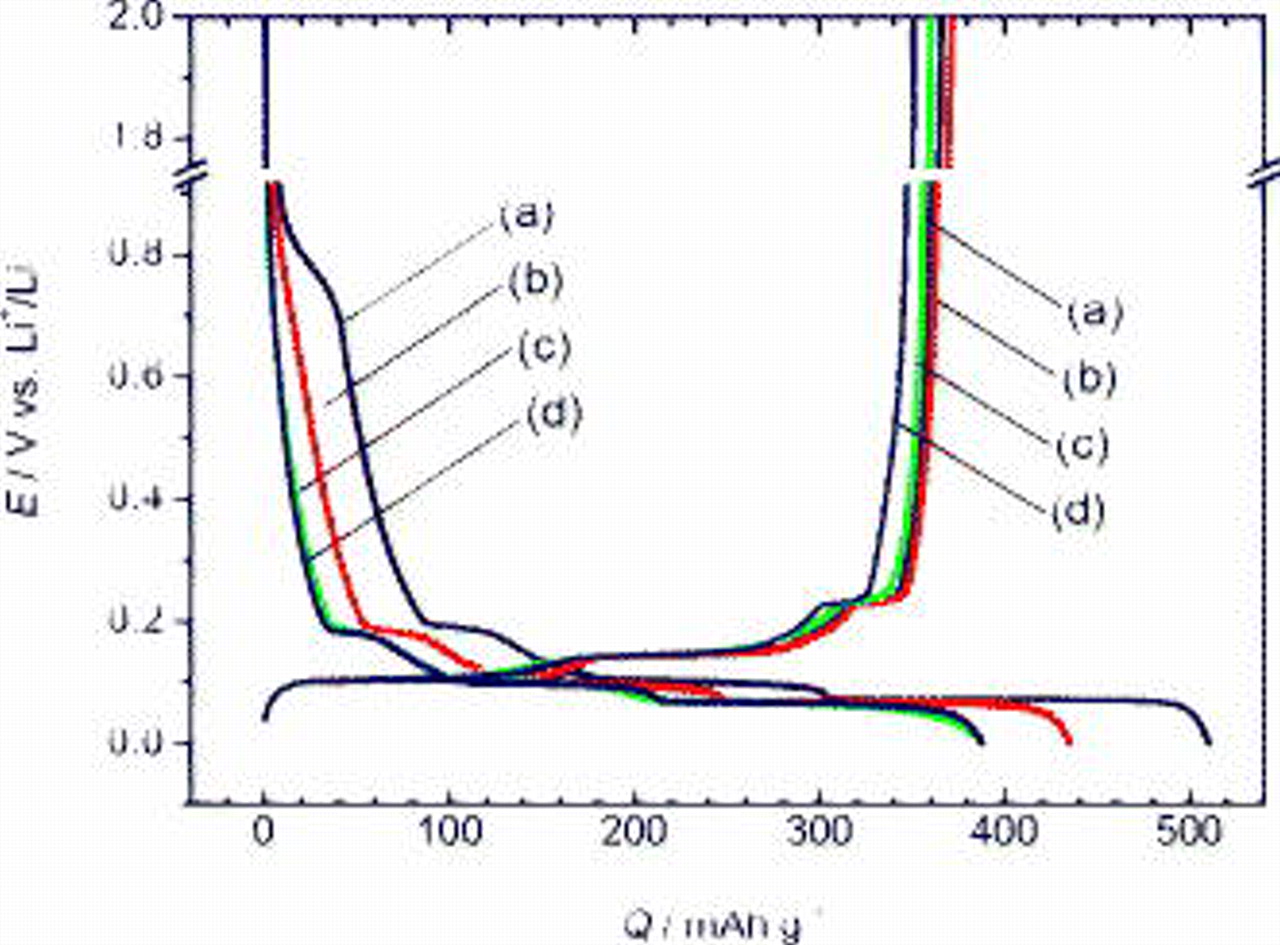
Figure 2 shows charge (electroreduction) and discharge (electro-oxidation) curves of graphite as a negative electrode at  in the EC:DMC electrolyte. Their potential variations seem to be similar, and several plateaus appear in the region between 0.3 and
in the EC:DMC electrolyte. Their potential variations seem to be similar, and several plateaus appear in the region between 0.3 and  vs Li, corresponding to the staging structure of Li-intercalated graphite.15 These plateaus are observed in both charge and discharge curves, proving the reversible lithium intercalation for the PVdF, PAAH, PAALi, and PAANa electrodes. Although the variations of potential in the discharge curves are quite similar for four electrodes, the charge curves are different because the irreversible capacities of the electrolyte decomposition, including the SEI formation, were different. In the case of PVdF, the irreversible capacity was observed around
vs Li, corresponding to the staging structure of Li-intercalated graphite.15 These plateaus are observed in both charge and discharge curves, proving the reversible lithium intercalation for the PVdF, PAAH, PAALi, and PAANa electrodes. Although the variations of potential in the discharge curves are quite similar for four electrodes, the charge curves are different because the irreversible capacities of the electrolyte decomposition, including the SEI formation, were different. In the case of PVdF, the irreversible capacity was observed around  . Clearly, the irreversible capacity for PVdF is larger than those of the other cases. For PAAH, the potential swiftly decreased in the initial stage of the first charge compared to PVdF. The irreversibility was remarkably suppressed by the use of PAALi and PAANa binders. The coulombic efficiencies at the initial cycle are 72, 85, 91, and 93% for PVdF, PAAH, PAALi, and PAANa, respectively. The efficiency of this parent graphite with PVdF seems rather low to be feasible in practical lithium-ion batteries, though the efficiency of the same graphite was confirmed to be higher in coin-type cells (Cu foil as current collector) instead of the beaker-type cells (Ni mesh as current collector) due to the different current distribution at the electrodes.15 It is likely that the improvement of efficiency was due to the chemical effect of the polyanion and alkali ions upon the electrolyte/electrode interface. The efficiency for PAAH (85%) was lower compared to that for PAANa and PAALi
. Clearly, the irreversible capacity for PVdF is larger than those of the other cases. For PAAH, the potential swiftly decreased in the initial stage of the first charge compared to PVdF. The irreversibility was remarkably suppressed by the use of PAALi and PAANa binders. The coulombic efficiencies at the initial cycle are 72, 85, 91, and 93% for PVdF, PAAH, PAALi, and PAANa, respectively. The efficiency of this parent graphite with PVdF seems rather low to be feasible in practical lithium-ion batteries, though the efficiency of the same graphite was confirmed to be higher in coin-type cells (Cu foil as current collector) instead of the beaker-type cells (Ni mesh as current collector) due to the different current distribution at the electrodes.15 It is likely that the improvement of efficiency was due to the chemical effect of the polyanion and alkali ions upon the electrolyte/electrode interface. The efficiency for PAAH (85%) was lower compared to that for PAANa and PAALi  in spite of the use of the same polyanion with the same polymerization degree.
in spite of the use of the same polyanion with the same polymerization degree.
Figure 2. Initial charge (electroreduction) and discharge (electro-oxidation) curves of graphite as a negative electrode with several binders, (a) PVdF, (b) PAAH, (c) PAALi, and (d) PAANa, in 
 EC:DMC at
EC:DMC at  .
.
As we described previously,20 polyacrylates are stable against electrochemical reduction as a polymer binder for negative electrode. Furthermore, graphite powders were uniformly covered with a polyacrylate thin layer, unlike PVdF; that is, the polyacrylates served as glue, so the polymer layers should partly act as a passivation film. On the contrary, the graphite surface was partly uncovered with PVdF binder, resulting in the larger area of direct contact between the graphite and electrolyte solution. From these observations, it is most likely that the carboxylic polymers were entrapped in the SEI layer formed by the electrolyte decomposition without decomposition of polyacrylate molecules.
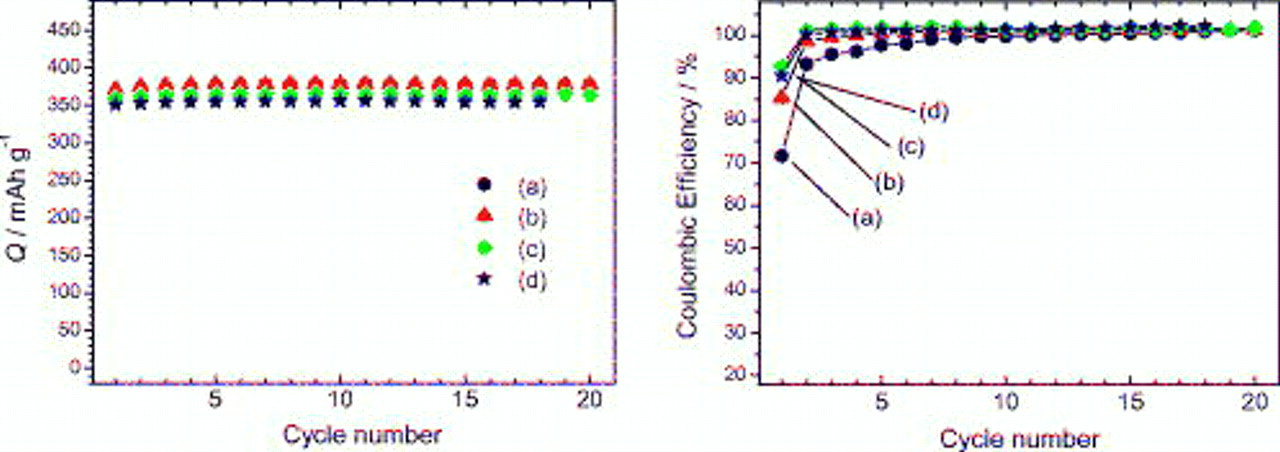
As seen in Fig. 3, the discharge capacities of about  , which is close to the electrochemical formation of
, which is close to the electrochemical formation of  , were maintained over
, were maintained over  with satisfactory efficiencies for all binders except for the first cycle. For PVdF, the efficiencies during the initial
with satisfactory efficiencies for all binders except for the first cycle. For PVdF, the efficiencies during the initial  were somewhat lower compared to those for PAAH, PAALi, and PAANa. As the potential plateaus corresponding to the stage structures were confirmed in charge and discharge curves over
were somewhat lower compared to those for PAAH, PAALi, and PAANa. As the potential plateaus corresponding to the stage structures were confirmed in charge and discharge curves over  , lithium intercalation into graphite and its reversibility did not suffer from not only PVdF but also PAAH, PAALi, and PAANa.
, lithium intercalation into graphite and its reversibility did not suffer from not only PVdF but also PAAH, PAALi, and PAANa.
Figure 3. Variation in discharge capacity (left) and coulombic efficiency (right) of negative graphite electrodes with several binders, (a) PVdF, (b) PAAH, (c) PAALi, and (d) PAANa, in 
 EC:DMC at
EC:DMC at  .
.
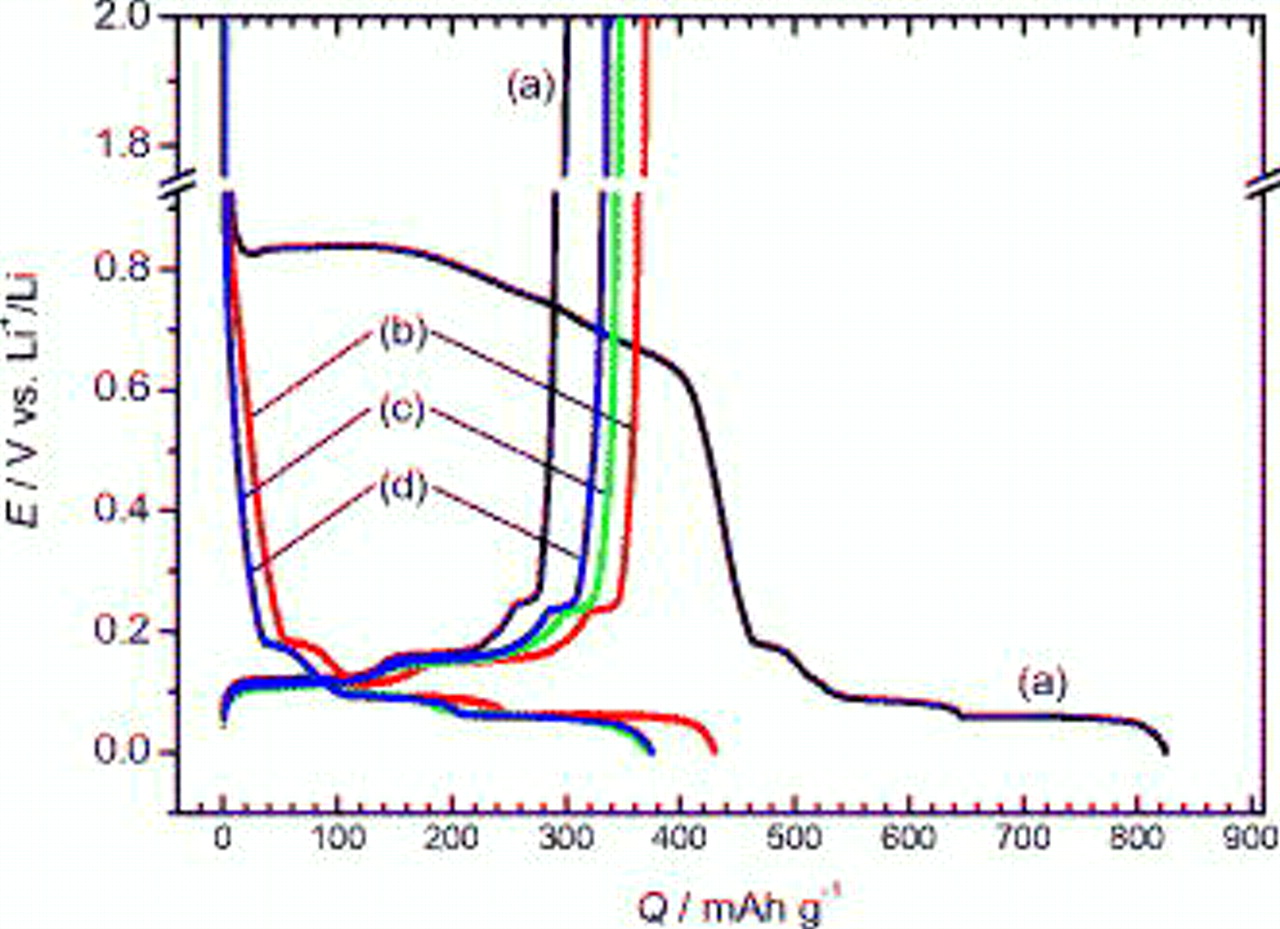
The same electrochemical tests were carried out in the EC-free PC media as shown in Fig. 4. Generally, PC-based electrolytes are known for their better low-temperature behavior compared with EC-based electrolytes due to the much lower freezing temperature of PC;24 therefore, they are attractive for practical lithium-ion cells. In the case of PVdF, the plateau with huge irreversible capacity appeared around  during the charge process in Fig. 4. The irreversible reaction accompanied by exfoliation of graphene layers due to massive cointercalation and decomposition of PC24, 25 is a well-known phenomenon which should occur during charge below ca.
during the charge process in Fig. 4. The irreversible reaction accompanied by exfoliation of graphene layers due to massive cointercalation and decomposition of PC24, 25 is a well-known phenomenon which should occur during charge below ca.  for PVdF. The exfoliation was confirmed by electron microscopy observation (not shown here). As a result, the severe irreversibility resulted in the low coulombic efficiency, 36% for PVdF electrode. As described by Buqa et al. ,26 the tolerance of graphite toward the exfoliation in PC-containing electrolyte increased for graphite with a high defect concentration. It is likely that the graphite used here has a relatively high defect concentration because of somewhat reversible intercalation of lithium, as shown in Fig. 4a.
for PVdF. The exfoliation was confirmed by electron microscopy observation (not shown here). As a result, the severe irreversibility resulted in the low coulombic efficiency, 36% for PVdF electrode. As described by Buqa et al. ,26 the tolerance of graphite toward the exfoliation in PC-containing electrolyte increased for graphite with a high defect concentration. It is likely that the graphite used here has a relatively high defect concentration because of somewhat reversible intercalation of lithium, as shown in Fig. 4a.
Figure 4. Charge and discharge curves of negative graphite electrodes with (a) PVdF, (b) PAAH, (c) PAALi, and (d) PAANa binders in 
 PC at
PC at  .
.
Surprisingly, the polyacrylate-modified graphite electrodes worked as a negative electrode in the EC-free PC electrolyte as well as in the EC:DMC electrolyte, as shown in Fig. 4. Their potential variations in the PC were almost identical to those in the EC:DMC in Fig. 2 and agree with the formation of the stage structure. Therefore, reversible lithium intercalation was achieved in the PC solution for the graphite bound not only with PAAH but also with PAALi and PAANa. Their efficiencies were 86, 93, and 90% for PAAH, PAALi, and PAANa, respectively.
In general, the cointercalation of PC molecules solvating  ion is considered to result in graphite expansion and collapse of the lamella structure. The role of SEI is important for the journey of solvated lithium ion from solution bulk to graphene interior as described in Ref. 27. Furthermore, when functional groups containing oxygen, such as carboxyl, hydroxyl, and carbonyl groups, were formed on graphite surface by chemical oxidation, the surface film containing
ion is considered to result in graphite expansion and collapse of the lamella structure. The role of SEI is important for the journey of solvated lithium ion from solution bulk to graphene interior as described in Ref. 27. Furthermore, when functional groups containing oxygen, such as carboxyl, hydroxyl, and carbonyl groups, were formed on graphite surface by chemical oxidation, the surface film containing  and
and  organic lithium salts was formed, resulting in stabilizing the graphite electrode and causing the capacity to increase.28 We believed that the carboxyl groups of the polyanion incorporated in the interphase during the electrolyte decomposition successfully enhanced the desolvation of EC- and PC-solvated
organic lithium salts was formed, resulting in stabilizing the graphite electrode and causing the capacity to increase.28 We believed that the carboxyl groups of the polyanion incorporated in the interphase during the electrolyte decomposition successfully enhanced the desolvation of EC- and PC-solvated  ions to reduce the initial irreversibility in both electrolytes. Namely, lithium ions in the PC should be successfully desolvated and intercalated with the help of the polyacryltate-immobilized SEI layer, as was recently discussed.19, 20 Further analyses of the interaction between polyacrylate and lithium ions at the interface are under progress and will be reported elsewhere in the near future.
ions to reduce the initial irreversibility in both electrolytes. Namely, lithium ions in the PC should be successfully desolvated and intercalated with the help of the polyacryltate-immobilized SEI layer, as was recently discussed.19, 20 Further analyses of the interaction between polyacrylate and lithium ions at the interface are under progress and will be reported elsewhere in the near future.
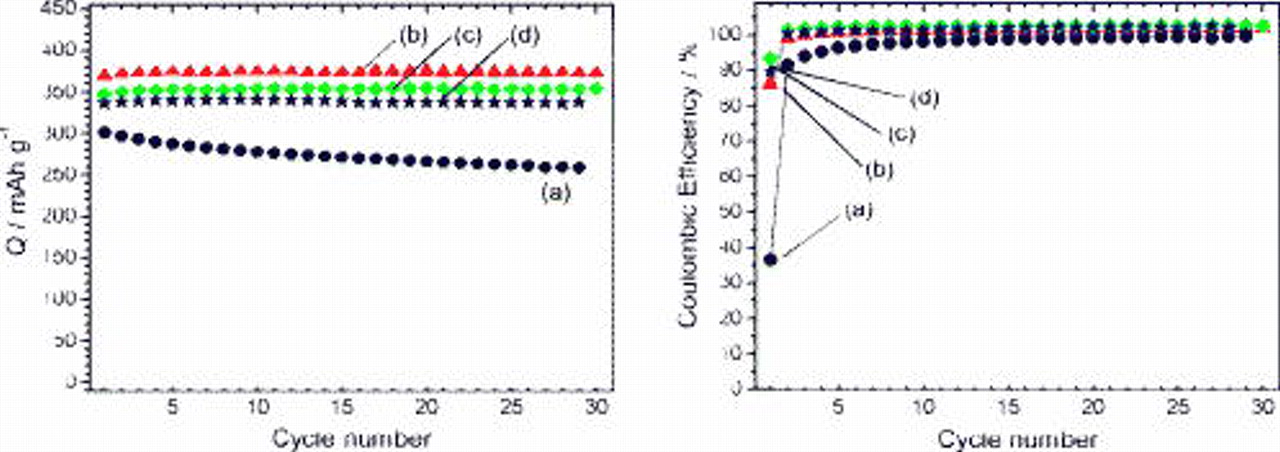
Figure 5 shows the dependence of discharge capacity and efficiency during extended cycles on the binding polymers. Although the PVdF electrode showed low capacity due to the decomposition accompanying graphite exfoliation, the polyacrylate anion modification achieved a reversible capacity of ca.  during successive cycling. Similar discharge capacities were obtained for PAAH, PAALi, and PAANa electrodes. These electrochemical results depended on the amount of polyacrylate binders, but the optimal loading of polyacrylate in the electrode was effective in the PC operation. We also investigated the relation between electrochemical performance of graphite and the polymerization degree of PAAH. The electrochemical results were almost independent of the polymerization degree, while the homogeneity of the slurry was required for the electrode fabrication.
during successive cycling. Similar discharge capacities were obtained for PAAH, PAALi, and PAANa electrodes. These electrochemical results depended on the amount of polyacrylate binders, but the optimal loading of polyacrylate in the electrode was effective in the PC operation. We also investigated the relation between electrochemical performance of graphite and the polymerization degree of PAAH. The electrochemical results were almost independent of the polymerization degree, while the homogeneity of the slurry was required for the electrode fabrication.
Figure 5. Variation in discharge capacity (left) and coulombic efficiency (right) of negative graphite electrodes with several binders, (a) PVdF, (b) PAAH, (c) PAALi, and (d) PAANa, in 
 PC at
PC at  .
.
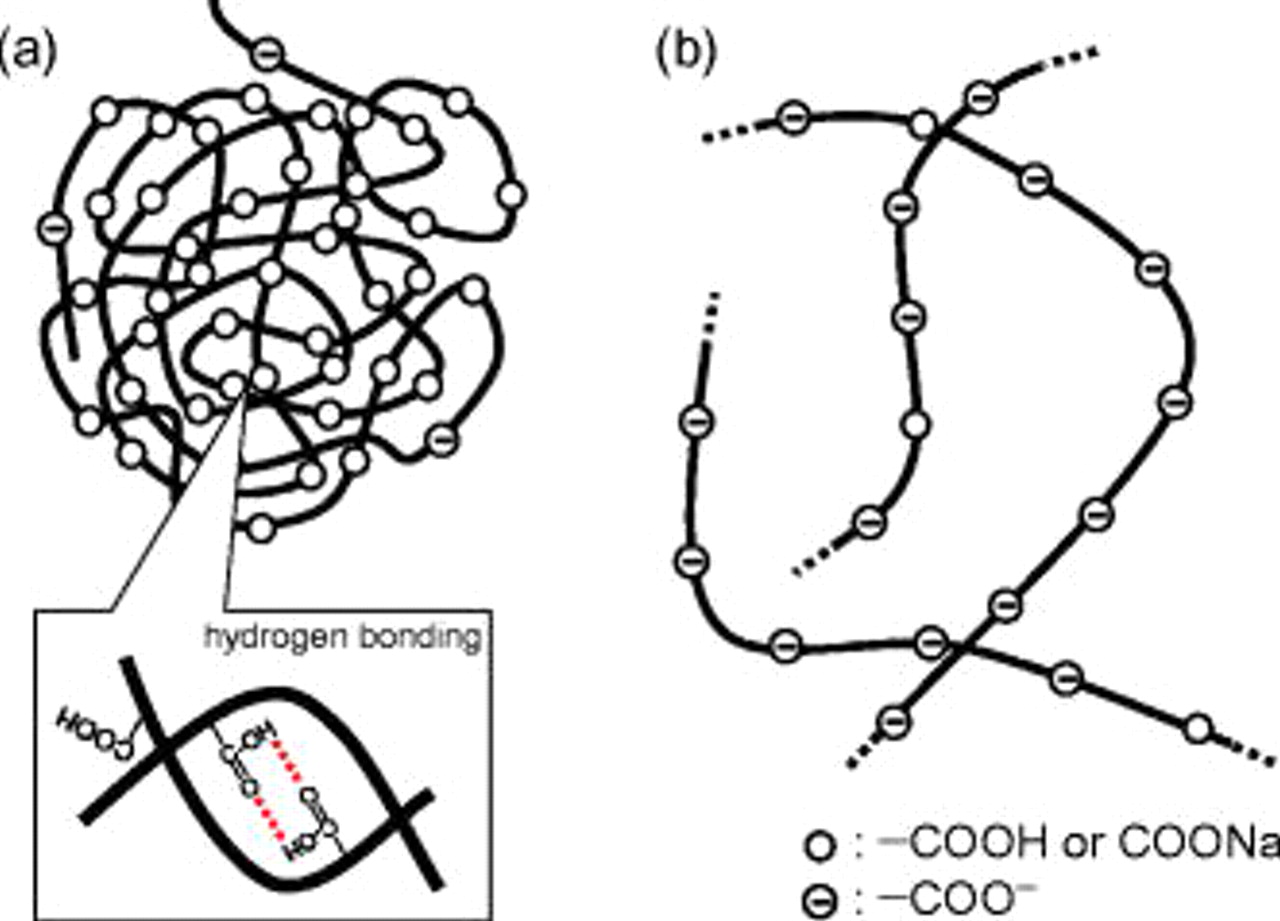
As mentioned above, the coulombic efficiencies in the EC:DMC electrolyte were 85, 91, and 93% for PAAH, PAALi, and PAANa, respectively. In the PC electrolyte, the efficiencies were 86, 93, and 90% for PAAH, PAALi, and PAANa, respectively. In both cases, the alkali polyacrylates led to higher efficiency than PAAH. We believe that this is due to the difference in polymer conformation as schematically drawn in Fig. 6. Generally, carboxyl groups of PAAH show weak acidity, and electrolytic dissociation is almost suppressed in a PAAH aqueous solution which is weakly acidic. Almost-neutral PAAH molecules, therefore, are prone to aggregate through hydrogen bonding between two carboxyl groups; ball-like conformation is drawn in Fig. 6a. Because hydrolysis of the alkali salts occurs in their aqueous solution, electrolytic dissociation of PAANa and PAALi is induced in aqueous solutions which are weakly basic. As a result, the polyacrylate chain is stretched due to the electrostatic repulsion between neighboring dissociated carboxy groups. The alkali polyacrylate solution possessed higher viscosity than the PAAH solution because of the difference in polymer conformation, that is, the stretched polyacrylate increases the flowage resistance of the aqueous solution. When graphite powder was pasted on the current collector with aqueous slurry, the slurry of PAALi and PAANa showed higher viscosity than that of PAAH. It is believed that graphite was coated with a uniform polyacrylate thin layer for PAALi and PAANa to dry the slurry; however, some island deposits of PAAH should be formed on graphite, resulting in a less-homogeneous layer of PAAH. It is reasonable to believe that the higher coulombic efficiency at the initial cycle in both EC:DMC and PC media was achieved by the uniform polyacrylate modification of graphite by use of PAALi and PAANa.
Figure 6. Schematic illustration of polymer chain conformation of (a) PAAH and (b) sodium polyacrylate dissolved in water.
The results of this study indicate that there is a general possibility of new interface functionality by binder polymers of graphite electrode. The interface modification of various intercalation electrodes by various binding polymers is expected to realize new intercalation activity, which in turn would lead to new redox reaction for advanced battery application.
Acknowledgments
This study was financially supported by the Iketani Science and Technology Foundation and the New Energy and Industrial Technology Development Organization (NEDO), Japan.
Tokyo University of Science assisted in meeting the publication costs of this article.