Abstract
Adiponitrile,  , ADN, was evaluated as both a solvent and cosolvent in safer and more electrochemically stable electrolytes suitable for high energy and power density Li-ion batteries. An electrochemical investigation of its electrolyte solution with the
, ADN, was evaluated as both a solvent and cosolvent in safer and more electrochemically stable electrolytes suitable for high energy and power density Li-ion batteries. An electrochemical investigation of its electrolyte solution with the  , LiTFSI, salt showed a wide electrochemical window of
, LiTFSI, salt showed a wide electrochemical window of  vs
vs  . The high melting point and the incompatibility of ADN with graphite anode required the use of ethylene carbonate (EC) as a cosolvent. The resultant EC:ADN electrolyte solutions showed ionic conductivities reaching
. The high melting point and the incompatibility of ADN with graphite anode required the use of ethylene carbonate (EC) as a cosolvent. The resultant EC:ADN electrolyte solutions showed ionic conductivities reaching  , viscosities of
, viscosities of  , and an improved resistance to aluminum corrosion up to
, and an improved resistance to aluminum corrosion up to  , all at
, all at  . Li-ion batteries incorporating graphite/
. Li-ion batteries incorporating graphite/ electrodes were assembled using EC:ADN electrolyte mixture containing
electrodes were assembled using EC:ADN electrolyte mixture containing  LiTFSI and
LiTFSI and  LiBOB as a cosalt, and discharge capacities of
LiBOB as a cosalt, and discharge capacities of  with very good capacity retention were obtained. AC impedance spectra of the batteries recorded as a function of charging and cycling indicated the presence of a stable solid electrolyte interface.
with very good capacity retention were obtained. AC impedance spectra of the batteries recorded as a function of charging and cycling indicated the presence of a stable solid electrolyte interface.
Export citation and abstract BibTeX RIS
Li-ion battery technology is the front-runner for application in the electric vehicle (EV), and its hybrid EV (HEV) and plug-in hybrid (PHEV) homologs.1, 2 This application is more demanding on the battery and requires an enhancement of its specifications particularly, increasing the power/energy densities and cycle life, and most importantly, enhancing its safety under normal and abusive operating conditions and of course lowering manufacturing cost.3, 4 One way to increase the energy density of the battery is to use cathode materials operating at high voltages  , that unfortunately current electrolytes based on organic carbonates cannot typically sustain. The carbonate-based electrolyte systems [e.g., ethylene carbonate (EC), dimethyl carbonate (DMC), diethyl carbonate (DEC)] function well in a Li-ion battery by virtue of their ability to form a stable solid electrolyte interface (SEI) on battery electrodes, as in the case in the
, that unfortunately current electrolytes based on organic carbonates cannot typically sustain. The carbonate-based electrolyte systems [e.g., ethylene carbonate (EC), dimethyl carbonate (DMC), diethyl carbonate (DEC)] function well in a Li-ion battery by virtue of their ability to form a stable solid electrolyte interface (SEI) on battery electrodes, as in the case in the  commercial battery that utilizes graphite/
commercial battery that utilizes graphite/ electrodes. Moreover, the use of higher voltage cathode materials that can support battery redox reactions as high as
electrodes. Moreover, the use of higher voltage cathode materials that can support battery redox reactions as high as  vs
vs  is possible, depending on the right choice of cathode and electrolyte (e.g.,
is possible, depending on the right choice of cathode and electrolyte (e.g.,  with EC:DEC), but it was found that the battery performance cannot be sustained on prolonged cycling at such high potentials.5 Furthermore, the carbonate system cannot be used with cathode materials working at higher redox potentials (e.g.,
with EC:DEC), but it was found that the battery performance cannot be sustained on prolonged cycling at such high potentials.5 Furthermore, the carbonate system cannot be used with cathode materials working at higher redox potentials (e.g.,  ),6 and is not safe under abusive conditions. One of the main issues being the solvents' low boiling and flash points and autoignition temperatures, and the salt's
),6 and is not safe under abusive conditions. One of the main issues being the solvents' low boiling and flash points and autoignition temperatures, and the salt's  low thermal stability and sensitivity to hydrolysis, all detrimental to the battery's components, function, and safety.
low thermal stability and sensitivity to hydrolysis, all detrimental to the battery's components, function, and safety.
High-voltage electrolytes based on solvents bearing the sulfone functional group,  -, were reported by Xu and Angell7, 8 and Sun and Angell.9 Wide electrochemical windows extending over
-, were reported by Xu and Angell7, 8 and Sun and Angell.9 Wide electrochemical windows extending over  were found for synthetic sulfones with various molecular structures. Unfortunately, high melting points and issues concerning compatibility with graphite (the ability to form a stable SEI) were found to be serious problems with the sulfone electrolyte system and therefore hampered their use as single solvents. Mixtures of two structurally different sulfone solvents (ones that are compatible with graphite, such as the fluorinated alkyls, and ones with low melting points, such as the oligoethers) and sulfone/carbonate electrolyte system were prepared, and although they have yet to be tested thoroughly in Li-ion batteries, the preliminary results on the fluorinated alkyls/carbonate system gave moderately good capacities in the initial battery cycles.8 Also, mixtures of sulfone and ester solvents were recently investigated in moderate- and high-voltage Li metal batteries, but poor cyclability was reported, the improvement of which was possible in the presence of organic additives.10
were found for synthetic sulfones with various molecular structures. Unfortunately, high melting points and issues concerning compatibility with graphite (the ability to form a stable SEI) were found to be serious problems with the sulfone electrolyte system and therefore hampered their use as single solvents. Mixtures of two structurally different sulfone solvents (ones that are compatible with graphite, such as the fluorinated alkyls, and ones with low melting points, such as the oligoethers) and sulfone/carbonate electrolyte system were prepared, and although they have yet to be tested thoroughly in Li-ion batteries, the preliminary results on the fluorinated alkyls/carbonate system gave moderately good capacities in the initial battery cycles.8 Also, mixtures of sulfone and ester solvents were recently investigated in moderate- and high-voltage Li metal batteries, but poor cyclability was reported, the improvement of which was possible in the presence of organic additives.10
The use of organic solvents based on the nitrile functional group,  , has been mainly limited to the simplest of all nitrile molecules, acetonitrile, which has been widely used in electrolytes for various electrochemical applications, including the
, has been mainly limited to the simplest of all nitrile molecules, acetonitrile, which has been widely used in electrolytes for various electrochemical applications, including the  battery, but its anodic stability in the presence of some lithium salts is limited to
battery, but its anodic stability in the presence of some lithium salts is limited to  . Moreover, other nitriles have been recently used in lithium metal and lithium-ion batteries utilizing succinonitrile with the
. Moreover, other nitriles have been recently used in lithium metal and lithium-ion batteries utilizing succinonitrile with the  anode11 or lithium metal anode12 and 3-methoxypropionitrile with the
anode11 or lithium metal anode12 and 3-methoxypropionitrile with the  anode.13 However, we are not aware of any reports of batteries involving the use of graphite as an anode due to its expected incompatibility with aliphatic nitrile monosolvents or disolvents.
anode.13 However, we are not aware of any reports of batteries involving the use of graphite as an anode due to its expected incompatibility with aliphatic nitrile monosolvents or disolvents.
In a study on liquid electrolytes for electrical double-layer capacitors, Ue et al.14, 15 demonstrated that electrolytes of tetraalkylammonium salts in dinitrile solvents,  , such as glutaronitrile (GLN)
, such as glutaronitrile (GLN)  and adiponitrile (ADN)
and adiponitrile (ADN)  exhibit resistance to electrochemical oxidation extending to
exhibit resistance to electrochemical oxidation extending to  vs saturated calomel electrode (
vs saturated calomel electrode ( vs
vs  ). This extra stability is higher than that of all aprotic solvents, including those based on the sulfone family.16–18
). This extra stability is higher than that of all aprotic solvents, including those based on the sulfone family.16–18
Besides the extra anodic stability, ADN and GLN show the best thermal (high boiling point and flash point) and physical (high dielectric constant and low viscosity) properties of all solvents in the dinitrile family. We conducted work on the characteristics and performance of lithium electrolytes based on the two solvents, but herein we report on the electrolyte system based on ADN. Li-ion batteries incorporating  LiTFSI in ADN along with graphite as an anode were tested. The lower intercalation/deintercalation voltage of
LiTFSI in ADN along with graphite as an anode were tested. The lower intercalation/deintercalation voltage of  ion in graphite
ion in graphite  than that of
than that of 
 , despite the latter being more compatible with nitrile solvents, in general, including ADN, renders the fabrication of higher voltage batteries and, therefore, it is used in this work and as will be reported the nitrile/graphite interface can be stabilized by the use of EC and LiBOB.
, despite the latter being more compatible with nitrile solvents, in general, including ADN, renders the fabrication of higher voltage batteries and, therefore, it is used in this work and as will be reported the nitrile/graphite interface can be stabilized by the use of EC and LiBOB.
We report here on the physical and electrochemical characteristics of lithium electrolyte solutions based on ADN alone or combined with ethylene carbonate, and their performance in Li-ion batteries incorporating conventional electrodes with graphite as anode.
Experimental
The electrolytes were prepared by dissolving the appropriate amount of the LiTFSI (lithium bis-trifluoromethanesulfonyl imide) salt  or LiBOB (lithium bis(oxalato)borate) (Chemmetal) into the solvent (Adiponitrile, Fluka) or solvent mixtures (ethylene carbonate, Aldrich) in the appropriate volume ratios. The electrolyte solutions were mixed well, and if necessary, heated until complete dissolution. Conductivity measurements were performed using the ac impedance spectroscopy technique for which the electrolyte solutions were poured into a two-platinum-electrode conductivity cell with a cell constant of 0.96. The frequency was swept between
or LiBOB (lithium bis(oxalato)borate) (Chemmetal) into the solvent (Adiponitrile, Fluka) or solvent mixtures (ethylene carbonate, Aldrich) in the appropriate volume ratios. The electrolyte solutions were mixed well, and if necessary, heated until complete dissolution. Conductivity measurements were performed using the ac impedance spectroscopy technique for which the electrolyte solutions were poured into a two-platinum-electrode conductivity cell with a cell constant of 0.96. The frequency was swept between  and
and  using a Solatron frequency analyzer (model no. 1260) and the temperature was varied between
using a Solatron frequency analyzer (model no. 1260) and the temperature was varied between  and
and  , allowing
, allowing  for thermal equilibration between measurements. Cyclic voltammograms were realized with a platinum microelectrode
for thermal equilibration between measurements. Cyclic voltammograms were realized with a platinum microelectrode  for the electrochemical window measurements or with an aluminum wire
for the electrochemical window measurements or with an aluminum wire  for corrosion studies, and a silver wire was used as a counter and pseudo reference electrode. The true potential was established with butyl-ferrocene (Aldrich) and was found at
for corrosion studies, and a silver wire was used as a counter and pseudo reference electrode. The true potential was established with butyl-ferrocene (Aldrich) and was found at  vs
vs  . The scan rate was
. The scan rate was  , and all measurements were conducted at ambient temperature.
, and all measurements were conducted at ambient temperature.
Battery investigations were carried out with coin-type (size 2325) cells. Cathode and anode materials were prepared by mixing 85:5:5:5 (w/w) ratios of active material, carbon black, super S, and poly(vinylidenefluoride) in  -methyl pyrrolidinone binder, respectively. The resulting paste was applied to an aluminum (
-methyl pyrrolidinone binder, respectively. The resulting paste was applied to an aluminum ( , SEIMI Chemical Co. Ltd.) or copper (MCMB, Osaka Gas Co.) foil current-collector and then was dried, first at
, SEIMI Chemical Co. Ltd.) or copper (MCMB, Osaka Gas Co.) foil current-collector and then was dried, first at  and then at
and then at  under vacuum for two days. A Celgard separator (
under vacuum for two days. A Celgard separator ( thickness) was placed between electrodes and soaked wet with the electrolyte. The cells were assembled in an Ar-filled dry box at room temperature. Cell performance was evaluated by galvanostatic experiments carried out on a multichannel Arbin battery cycler. The cells were first charged and then discharged at a constant current density (C/12) between 2.5 and
thickness) was placed between electrodes and soaked wet with the electrolyte. The cells were assembled in an Ar-filled dry box at room temperature. Cell performance was evaluated by galvanostatic experiments carried out on a multichannel Arbin battery cycler. The cells were first charged and then discharged at a constant current density (C/12) between 2.5 and  . Differential scanning calorimetric analysis was performed using a TA Instruments 2920. All the samples were sealed in aluminum pans inside an Argon-filled glove box and then scanned from
. Differential scanning calorimetric analysis was performed using a TA Instruments 2920. All the samples were sealed in aluminum pans inside an Argon-filled glove box and then scanned from  at
at  rate. Viscosity measurements were conducted using a Cannon viscometer (E455, 100). All experiments and handling of chemicals were performed in an Ar-filled glove box.
rate. Viscosity measurements were conducted using a Cannon viscometer (E455, 100). All experiments and handling of chemicals were performed in an Ar-filled glove box.
Results and Discussion
Electrochemical stability of the electrolytes
Adiponitrile is a colorless, moderately viscous  liquid with a dielectric constant
liquid with a dielectric constant  of 30. Electrolyte solutions of ADN and the lithium salt of the noncoordinating imide anion bis(trifluoromethanesulfonyl)imide (TFSI), LiTFSI, were prepared and the salt dissolved readily over all concentrations. The
of 30. Electrolyte solutions of ADN and the lithium salt of the noncoordinating imide anion bis(trifluoromethanesulfonyl)imide (TFSI), LiTFSI, were prepared and the salt dissolved readily over all concentrations. The  electrolyte solution was used to record the cyclic voltammetry (CV) scan on a Pt microelectrode
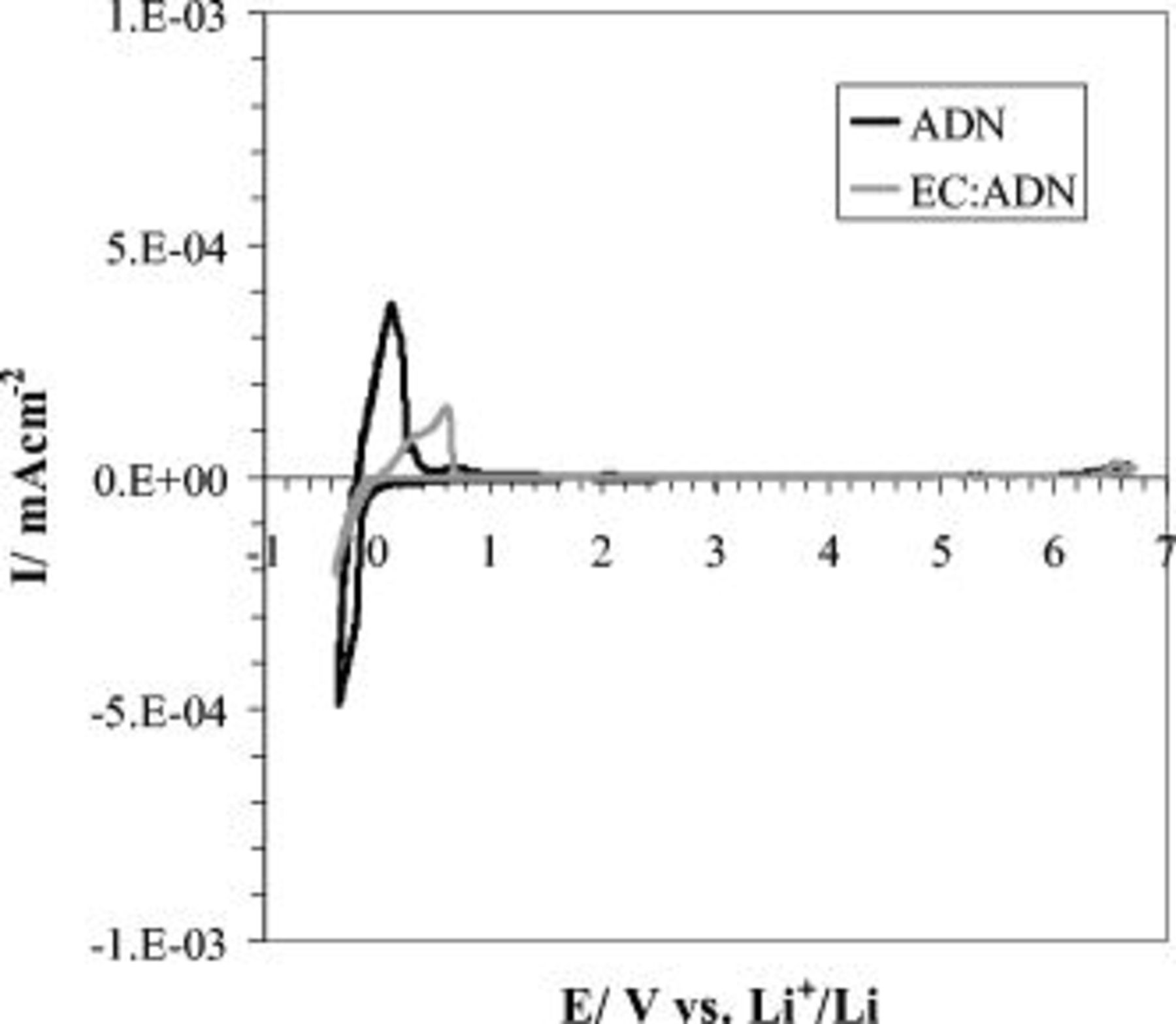
electrolyte solution was used to record the cyclic voltammetry (CV) scan on a Pt microelectrode  and is shown in Fig. 1. The scan indicates that there is an electrochemical window of
and is shown in Fig. 1. The scan indicates that there is an electrochemical window of  within which there are no apparent oxidation peak currents, which is much higher than what is reported for carbonate,4 sulfone,7 and ionic liquid19 electrolyte systems. The limit on the oxidation potential of the cyclic voltammogram is not due to the nitrile oxidation but rather to that of the imide anion, which is known to be less stable compared to other delocalized anions, such as
within which there are no apparent oxidation peak currents, which is much higher than what is reported for carbonate,4 sulfone,7 and ionic liquid19 electrolyte systems. The limit on the oxidation potential of the cyclic voltammogram is not due to the nitrile oxidation but rather to that of the imide anion, which is known to be less stable compared to other delocalized anions, such as  ,20 the use of which is limited by the poor solubility of
,20 the use of which is limited by the poor solubility of  in adiponitrile.
in adiponitrile.
Figure 1. CV scans of  LiTFSI electrolyte solutions of ADN and EC:ADN (1:1 by volume) recorded on a Pt electrode
LiTFSI electrolyte solutions of ADN and EC:ADN (1:1 by volume) recorded on a Pt electrode  at a scan rate of
at a scan rate of  .
.
The extraordinary anodic stability of adiponitrile can be explained in terms of the unique molecular structure of the nitrile group,21 because it has a triple bond of  and two perpendicular π bonds between the C and N atoms, which results in a very strong bond
and two perpendicular π bonds between the C and N atoms, which results in a very strong bond  that have tightly bound electrons locked in low-energy-level highest-occupied molecular orbitals, rendering their transfer to the Fermi level of metal electrodes difficult.22 Furthermore, the presence of four methylene groups that are electron donating to the two nitrile groups adds more stability to ADN.
that have tightly bound electrons locked in low-energy-level highest-occupied molecular orbitals, rendering their transfer to the Fermi level of metal electrodes difficult.22 Furthermore, the presence of four methylene groups that are electron donating to the two nitrile groups adds more stability to ADN.
Because of the high melting point of adiponitrile  , which limits its use in batteries operating at lower temperatures, there is a need to use a cosolvent, the choice of which involved screening a wide variety of carbonate and other nitrile solvents. We have chosen EC due to its high dielectric constant, low viscosity in the molten state, very good thermal properties almost comparable to adiponitrile, and its excellent performance in Li-ion batteries using graphite as an anode due to its ability to form a compact, functioning SEI. This is very important in our case because it is well known that aliphatic nitrile solvents are prone to ease of reduction in the presence of graphite and the SEI formed by the reduction product of EC will help protect the nitrile component of the electrolyte.23 The solution mixture of the two solvents (1:1 by volume) with LiTFSI
, which limits its use in batteries operating at lower temperatures, there is a need to use a cosolvent, the choice of which involved screening a wide variety of carbonate and other nitrile solvents. We have chosen EC due to its high dielectric constant, low viscosity in the molten state, very good thermal properties almost comparable to adiponitrile, and its excellent performance in Li-ion batteries using graphite as an anode due to its ability to form a compact, functioning SEI. This is very important in our case because it is well known that aliphatic nitrile solvents are prone to ease of reduction in the presence of graphite and the SEI formed by the reduction product of EC will help protect the nitrile component of the electrolyte.23 The solution mixture of the two solvents (1:1 by volume) with LiTFSI  was prepared, and its electrochemical stability was evaluated and the CV scan, also in Fig. 1, showed similar electrochemical stability to the single ADN electrolyte.
was prepared, and its electrochemical stability was evaluated and the CV scan, also in Fig. 1, showed similar electrochemical stability to the single ADN electrolyte.
Conductivity and viscosity of the electrolytes
The conductivity of electrolyte solutions of  LiTFSI in ADN alone and with EC as a cosolvent were measured using an ac impedance spectroscopy technique between
LiTFSI in ADN alone and with EC as a cosolvent were measured using an ac impedance spectroscopy technique between  and
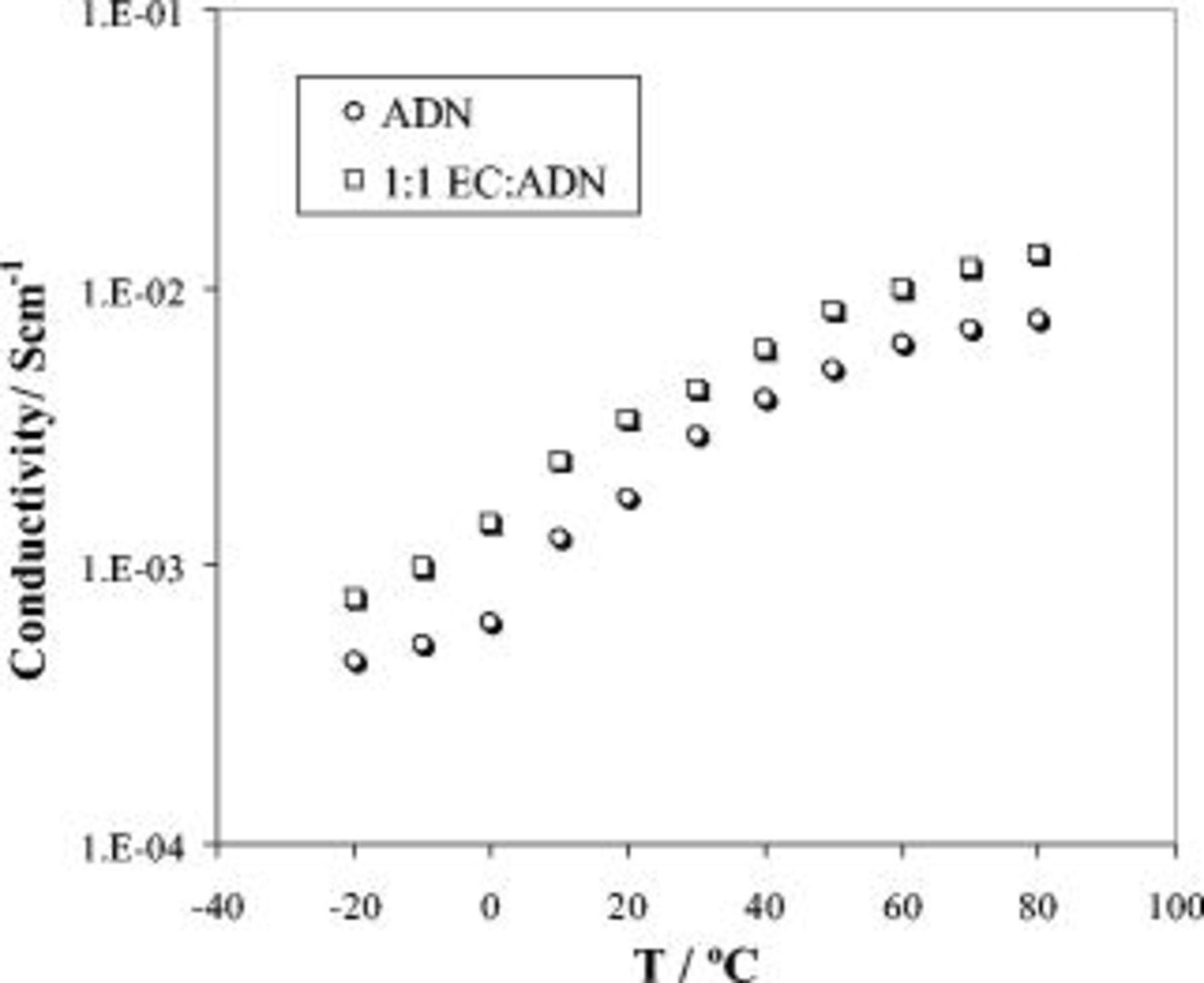
and  , and the results are shown in Fig. 2. The conductivity of the ADN electrolyte increased from
, and the results are shown in Fig. 2. The conductivity of the ADN electrolyte increased from  at
at  at
at  and reached
and reached  at
at  . When EC was mixed with ADN in 1:1 by volume ratio, a significant increase in the conductivity was observed even at low temperatures. It increased from
. When EC was mixed with ADN in 1:1 by volume ratio, a significant increase in the conductivity was observed even at low temperatures. It increased from  at
at  at
at  and reached
and reached  at
at  . The effect of adding the high dielectric constant EC (89.6) on the bulk conductivity lies in its ability to increase the number of dissociated ions "
. The effect of adding the high dielectric constant EC (89.6) on the bulk conductivity lies in its ability to increase the number of dissociated ions " ,
,  , or others" in the solvent mixture by increasing its dielectric constant to values presumably higher than that of ADN, and in enhancing the mobility of those ions by decreasing the viscosity of the solvent mixture, as shown below, and hence, the increase in the bulk conductivity of the solvent mixture electrolytes compared to the single ADN electrolyte.
, or others" in the solvent mixture by increasing its dielectric constant to values presumably higher than that of ADN, and in enhancing the mobility of those ions by decreasing the viscosity of the solvent mixture, as shown below, and hence, the increase in the bulk conductivity of the solvent mixture electrolytes compared to the single ADN electrolyte.
Figure 2. Conductivity as a function of temperature of  LiTFSI electrolyte solutions in ADN and its EC solutions.
LiTFSI electrolyte solutions in ADN and its EC solutions.
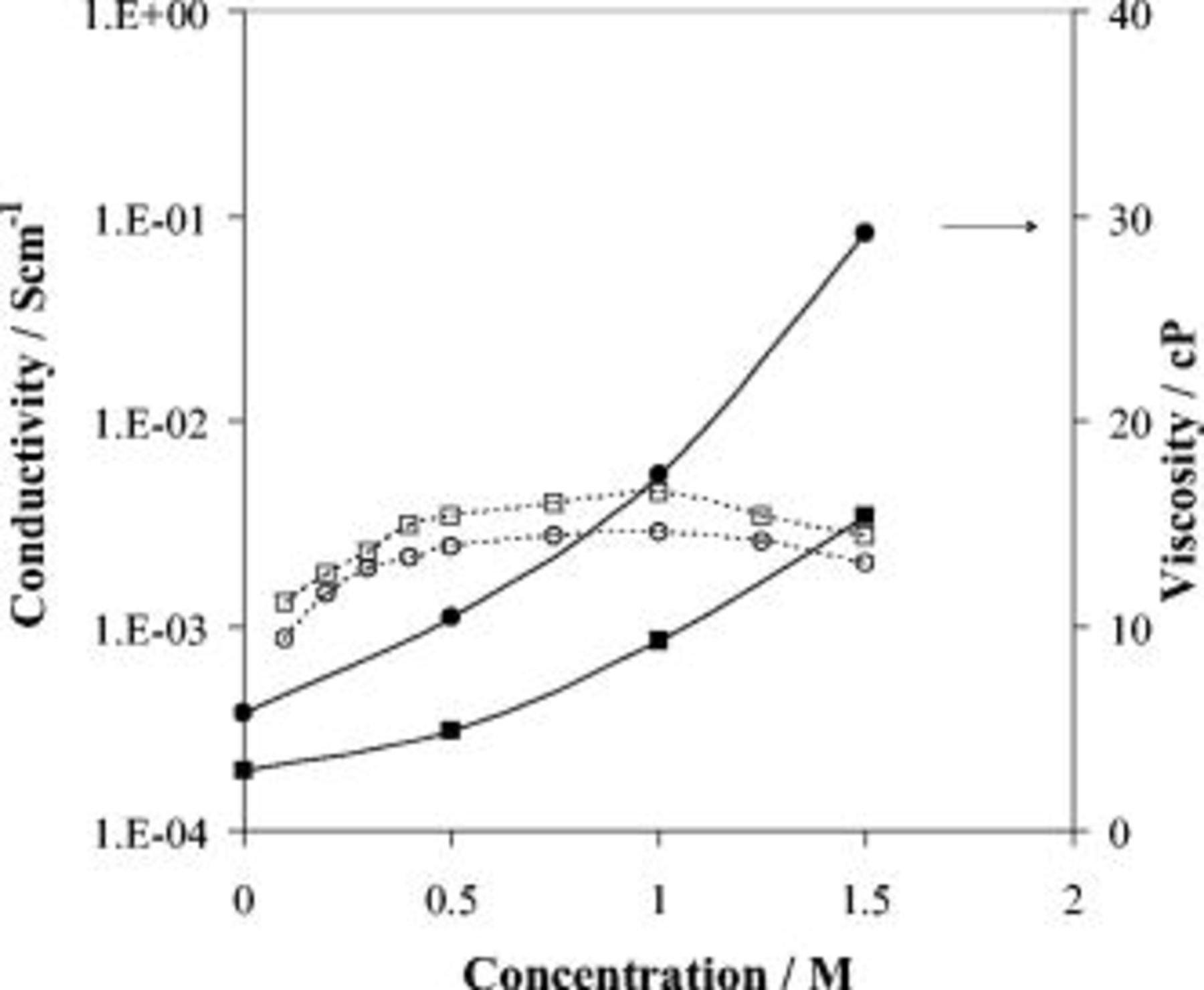
Figure 3 shows the conductivity of ADN and EC:ADN electrolytes as a function of LiTFSI concentration. The curve exhibits a typical behavior with the conductivity increasing gradually at low concentrations due to the increase in the number of charge carriers, going through a maximum in conductivity, in this case at  . At the high-concentration end, the conductivity decreased as a result of the formation of a more congested, compact environment around the ionic charge carriers that translates into the formation of the less mobile or neutral ion pairs and also an overall increase in viscosity.
. At the high-concentration end, the conductivity decreased as a result of the formation of a more congested, compact environment around the ionic charge carriers that translates into the formation of the less mobile or neutral ion pairs and also an overall increase in viscosity.
Figure 3. Conductivity and viscosity (filled) as a function of concentration of LiTFSI in ADN (squares), EC:ADN (circles) solutions.
The viscosity of the electrolytes was measured as a function of concentration and is also shown in Fig. 3. The viscosity of the ADN solvent was found to be  , a value that increased to
, a value that increased to  by the addition of LiTFSI
by the addition of LiTFSI  and even higher to
and even higher to  for the
for the  solution explaining partly the decrease in the high-concentration end of the conductivity. We also separately investigated the effect of EC on the viscosity of ADN and its electrolytes and found that the viscosity of ADN decreased from
solution explaining partly the decrease in the high-concentration end of the conductivity. We also separately investigated the effect of EC on the viscosity of ADN and its electrolytes and found that the viscosity of ADN decreased from  upon the addition of EC in 1:1 by volume. The addition of the LiTFSI salt to the mixture has shown a drastic increase in their viscosity, as it increased to
upon the addition of EC in 1:1 by volume. The addition of the LiTFSI salt to the mixture has shown a drastic increase in their viscosity, as it increased to  for the
for the  concentration and to
concentration and to  for the more concentrated
for the more concentrated  solution, which implies the presence of strong solvent-solvent and different types of ion-solvent interactions, resulting in structural changes in the solvated ions environment and more restrictions on their mobility. We can see from the lower viscosity/higher conductivity of the EC:ADN solvent mixture compared to single ADN electrolytes that EC, which is a solid at room temperature but when molten has a viscosity of
solution, which implies the presence of strong solvent-solvent and different types of ion-solvent interactions, resulting in structural changes in the solvated ions environment and more restrictions on their mobility. We can see from the lower viscosity/higher conductivity of the EC:ADN solvent mixture compared to single ADN electrolytes that EC, which is a solid at room temperature but when molten has a viscosity of  , has a diluting effect on the bulk viscosity, which decreased from
, has a diluting effect on the bulk viscosity, which decreased from  , resulting in an increase in the mobility of ions whose number has also been increased because of the EC addition, making electrolyte mixtures with superior conductivities.
, resulting in an increase in the mobility of ions whose number has also been increased because of the EC addition, making electrolyte mixtures with superior conductivities.
DSC scans of the electrolytes
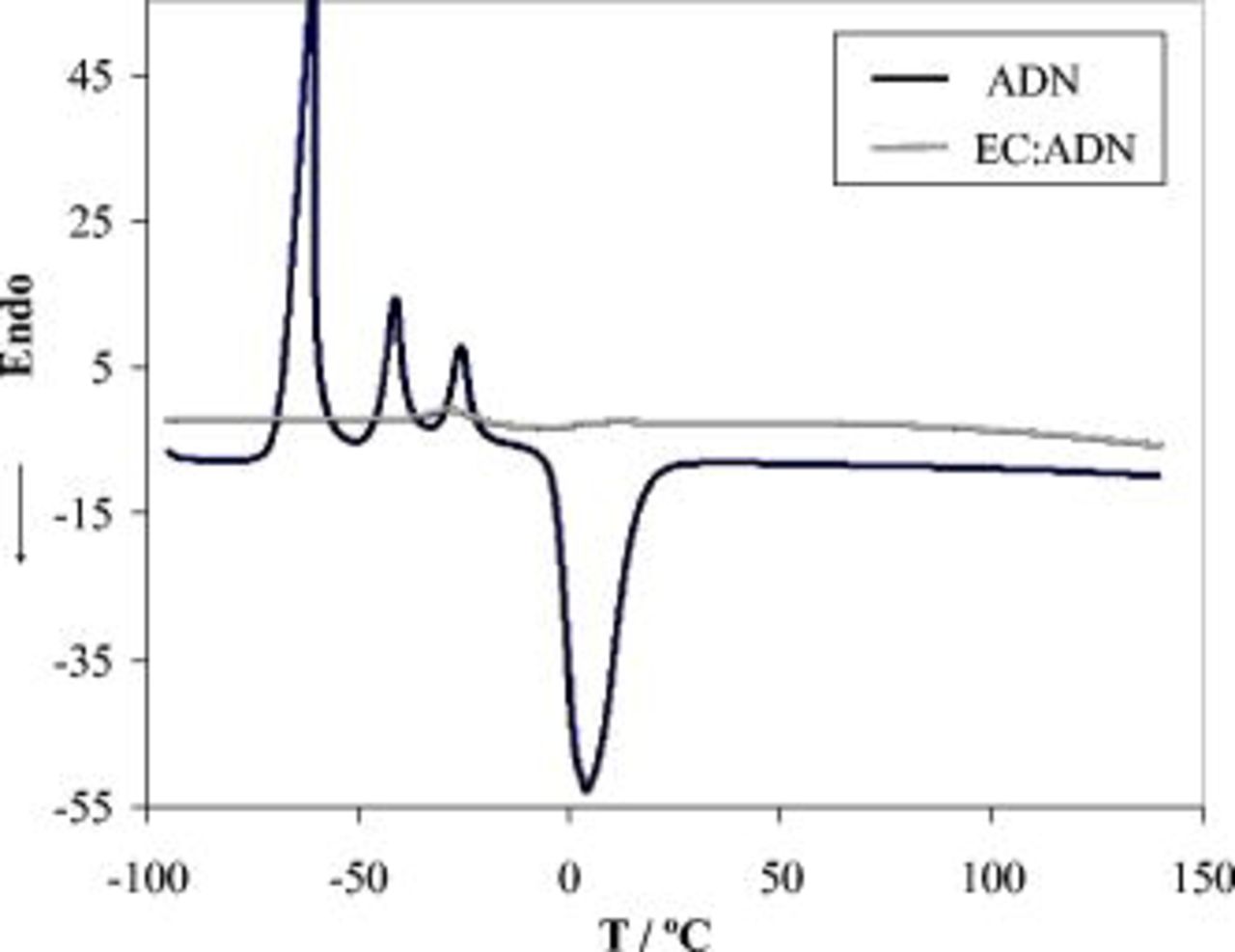
Figure 4 shows the differential scanning calorimetry (DSC) curves of ADN and EC:ADN (1:1 by volume). It can be observed that the ADN shows an endothermic peak at  corresponding to melting and three exothermic peaks at low temperatures that might be attributed to crystallization of salt/solvent complexes formed with different compositions. The affinity of the nitrile group to metal ions is well known, and previous reports on complex formation between lithium salts with acetonitrile or succinonitrile proved the existence of compounds of four cyano groups coordinating a Li cation, such as
corresponding to melting and three exothermic peaks at low temperatures that might be attributed to crystallization of salt/solvent complexes formed with different compositions. The affinity of the nitrile group to metal ions is well known, and previous reports on complex formation between lithium salts with acetonitrile or succinonitrile proved the existence of compounds of four cyano groups coordinating a Li cation, such as  24 or
24 or  12 complexes. Most importantly, the 1:1 curve showed no peaks in the temperature range between
12 complexes. Most importantly, the 1:1 curve showed no peaks in the temperature range between  and
and  , implying a complete dissolution between EC and ADN and a greater thermal stability when compared to carbonate-based electrolytes.
, implying a complete dissolution between EC and ADN and a greater thermal stability when compared to carbonate-based electrolytes.
Figure 4. DSC scans of  LiTFSI electrolyte solutions of ADN and EC:ADN (1:1 by volume).
LiTFSI electrolyte solutions of ADN and EC:ADN (1:1 by volume).
Battery performance
Li-ion batteries using conventional electrodes, mesocarbon microbead (MCMB) graphite and  , were assembled and tested in order to evaluate the performance of the electrolytes, and the results are shown in Fig. 5a. Batteries using
, were assembled and tested in order to evaluate the performance of the electrolytes, and the results are shown in Fig. 5a. Batteries using  LITFSI in ADN alone showed an initial discharge capacity of
LITFSI in ADN alone showed an initial discharge capacity of  that deteriorated gradually to have no capacity at all by the 40th cycle. This behavior is expected as described earlier in the text due to the incompatibility of ADN and graphite. LiBOB is a lithium battery electrolyte salt widely touted for its ability to form a good solid electrolyte interface. When LiBOB was added as a cosalt
that deteriorated gradually to have no capacity at all by the 40th cycle. This behavior is expected as described earlier in the text due to the incompatibility of ADN and graphite. LiBOB is a lithium battery electrolyte salt widely touted for its ability to form a good solid electrolyte interface. When LiBOB was added as a cosalt  to the electrolyte (we found that it has a limited solubility of
to the electrolyte (we found that it has a limited solubility of  in ADN), the initial discharge capacity was increased to
in ADN), the initial discharge capacity was increased to  , but on cycling the battery lost 80% of its capacity very rapidly in the first
, but on cycling the battery lost 80% of its capacity very rapidly in the first  , then stabilized at the same capacity up until the 50th cycle. When EC was used as a cosolvent (1:1 by volume), without any addition of LiBOB, the battery showed an initial capacity of
, then stabilized at the same capacity up until the 50th cycle. When EC was used as a cosolvent (1:1 by volume), without any addition of LiBOB, the battery showed an initial capacity of  that decreased gradually with 10% loss of capacity by the 20th cycle, and on cycling further, it decreased significantly reaching low values of
that decreased gradually with 10% loss of capacity by the 20th cycle, and on cycling further, it decreased significantly reaching low values of  by the 50th cycle.
by the 50th cycle.
Figure 5. (a) Effect of electrolyte components on the cycling performance of ADN-based electrolytes at C/12 in  batteries. (b) Specific capacity as a function of discharge current (C-Rate) for a MCMB/(
batteries. (b) Specific capacity as a function of discharge current (C-Rate) for a MCMB/( LiTFSI.
LiTFSI.  LiBOB) EC:ADN (1:1 by volume)/
LiBOB) EC:ADN (1:1 by volume)/ battery.
battery.
Finally, when EC was added in 1:1 by volume to ADN using the mixed salt electrolyte ( LiTFSI,
LiTFSI,  LiBOB), the battery exhibited excellent performance by showing an initial discharge capacity of
LiBOB), the battery exhibited excellent performance by showing an initial discharge capacity of  that, on cycling the battery further, showed very good capacity retention (90%) up to the
that, on cycling the battery further, showed very good capacity retention (90%) up to the  . The necessity of having both EC (can be reduced at
. The necessity of having both EC (can be reduced at  vs
vs  ) and LiBOB (its BOB anion can be reduced at
) and LiBOB (its BOB anion can be reduced at  vs
vs  ) at the same time in the electrolyte, and their role in the formation of a compact, stable "functioning" SEI has been previously demonstrated by Xu et al. who showed that the use of LiBOB alone does not result in a functioning SEI, especially at high temperatures, and that its reduction product and that of EC allow for the formation of a functional, multicomponent SEI leading to a good battery performance.25
) at the same time in the electrolyte, and their role in the formation of a compact, stable "functioning" SEI has been previously demonstrated by Xu et al. who showed that the use of LiBOB alone does not result in a functioning SEI, especially at high temperatures, and that its reduction product and that of EC allow for the formation of a functional, multicomponent SEI leading to a good battery performance.25
Figure 5b shows the discharge capacity of the batteries at room temperature as a function of discharge current (or C rate) for the battery using the electrolyte composed of 1:1 (EC:ADN by volume),  LiTFSI, and
LiTFSI, and  LiBOB. An increase in the C rate results in a gradual decrease in the discharge capacity of the batteries while it shows little effect on the discharge capacity after going through
LiBOB. An increase in the C rate results in a gradual decrease in the discharge capacity of the batteries while it shows little effect on the discharge capacity after going through  . The most profound decrease in the capacity (45% loss) was observed at the highest-tested discharge rate (C), which could be attributed to the higher viscosity (lower conductivity) of the electrolytes and possibly a more resistive interface compared to the carbonate system.
. The most profound decrease in the capacity (45% loss) was observed at the highest-tested discharge rate (C), which could be attributed to the higher viscosity (lower conductivity) of the electrolytes and possibly a more resistive interface compared to the carbonate system.
AC impedance spectroscopy of the batteries
AC impedance measurements were performed on the batteries with highest capacities and best capacity retention, which is the one with the 1:1 (EC: ADN by volume),  LiTFSI,
LiTFSI,  LiBOB electrolyte as a function of cycle number and state of charge. A typical spectrum of the battery shows a bulk (electrolyte, separator, electrodes, and current collectors) resistance
LiBOB electrolyte as a function of cycle number and state of charge. A typical spectrum of the battery shows a bulk (electrolyte, separator, electrodes, and current collectors) resistance  at the highest frequencies, two overlapped semicircles in the high- and middle-frequency regions, and an inclined line at the low-frequency region, all of which can be fitted using an equivalent circuit as the one shown in Fig. 6. The first of the semicircles corresponds to the SEI, the film formed on the electrodes/electrolyte interface
at the highest frequencies, two overlapped semicircles in the high- and middle-frequency regions, and an inclined line at the low-frequency region, all of which can be fitted using an equivalent circuit as the one shown in Fig. 6. The first of the semicircles corresponds to the SEI, the film formed on the electrodes/electrolyte interface  and its capacitance
and its capacitance  , while the second corresponds to the charge transfer resistance
, while the second corresponds to the charge transfer resistance  and its related double-layer capacitance
and its related double-layer capacitance  . The inclined line corresponds to the diffusion process of
. The inclined line corresponds to the diffusion process of  ions across the electrode/electrolyte interface.
ions across the electrode/electrolyte interface.
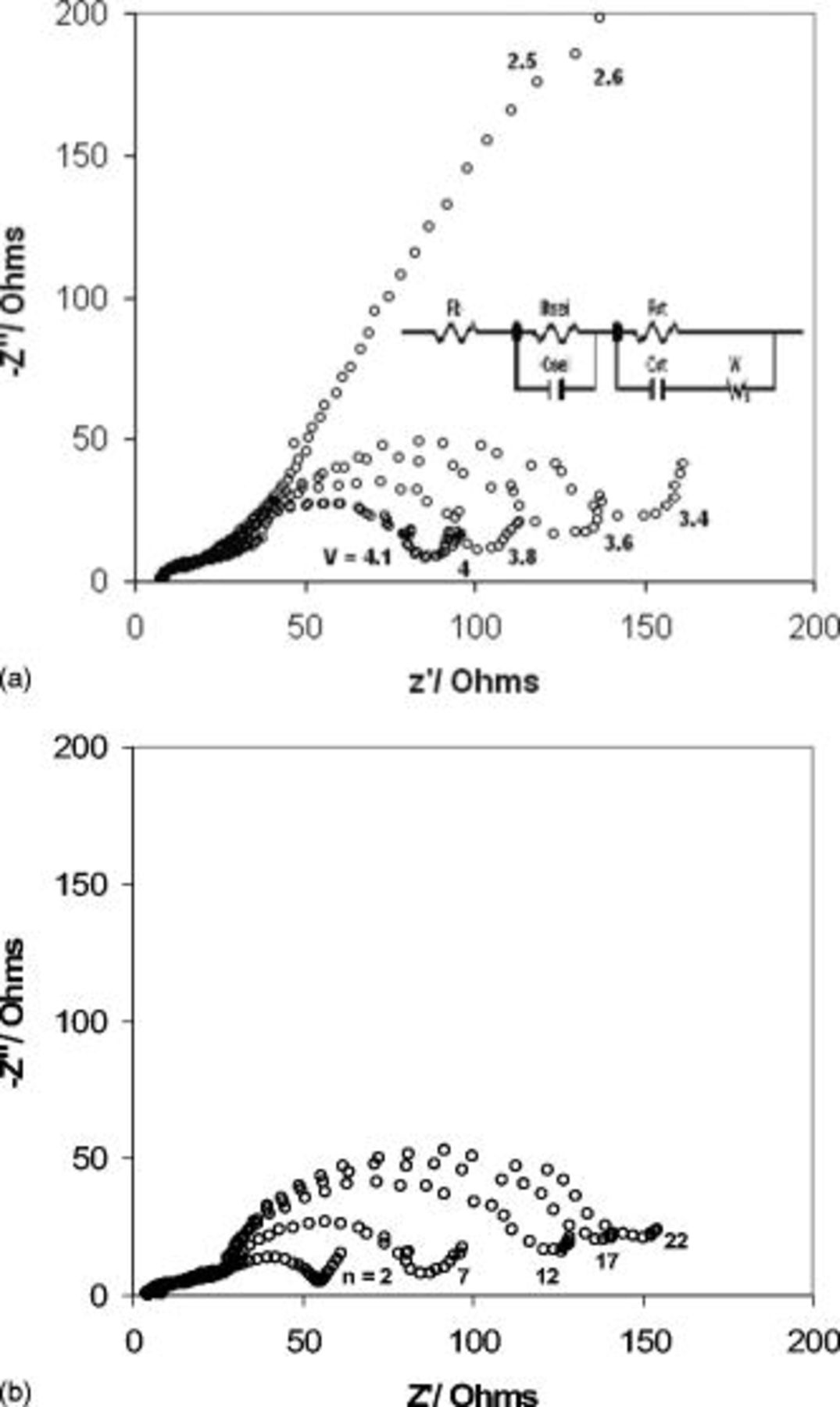
Figure 6. AC impedance spectra of a MCMB/( LiTFSI.
LiTFSI.  LiBOB) EC:ADN (1:1 by volume)/
LiBOB) EC:ADN (1:1 by volume)/ battery. (a) Impedance spectra of the battery during the seventh cycle as a function of the state of charge. (b) Impedance spectra of the battery as a function of cycle number.
battery. (a) Impedance spectra of the battery during the seventh cycle as a function of the state of charge. (b) Impedance spectra of the battery as a function of cycle number.
Figure 6 also shows the spectra of a Li-ion battery utilizing the EC:ADN electrolyte (1:1 by volume,  LiTFSI,
LiTFSI,  LiBOB) recorded at different voltages, between the discharged state at
LiBOB) recorded at different voltages, between the discharged state at  and the charged stage at
and the charged stage at  during the seventh cycle. It can be seen from Fig. 6 that during the charging process the
during the seventh cycle. It can be seen from Fig. 6 that during the charging process the  and
and  resistance values, measured to be
resistance values, measured to be  and
and  , respectively, are independent of the state of charge of the battery, a behavior similar to what is reported for carbonate-based solvents with a functional, stable SEI having high conductivity/diffusivity for Li ions.26 The
, respectively, are independent of the state of charge of the battery, a behavior similar to what is reported for carbonate-based solvents with a functional, stable SEI having high conductivity/diffusivity for Li ions.26 The  decreased significantly from a very high value estimated to be
decreased significantly from a very high value estimated to be  , due to the delithiation of the graphite anode, at
, due to the delithiation of the graphite anode, at  at
at  and further to
and further to  at
at  . Moreover, during the first charging cycle, the battery showed an overlapped semicircle with
. Moreover, during the first charging cycle, the battery showed an overlapped semicircle with  value of
value of  that decreased on the second cycle to
that decreased on the second cycle to  , corresponding to the formation of the SEI.
, corresponding to the formation of the SEI.
The impedance spectra was also monitored during the cycling process, where measurements were taken each cycle at the charged  and discharged
and discharged  stages and are presented in Fig. 6. A similar behavior was obtained during cycling of the battery except for the first and second cycles.
stages and are presented in Fig. 6. A similar behavior was obtained during cycling of the battery except for the first and second cycles.  and
and  were the same and stayed invariable, and
were the same and stayed invariable, and  increased gradually from
increased gradually from  at the second cycle to
at the second cycle to  at the 22nd cycle. This increase in
at the 22nd cycle. This increase in  , which can be related to the electrode kinetics and is therefore responsible for the capacity loss during cycling, could be related to passive layer formation or structural changes on the
, which can be related to the electrode kinetics and is therefore responsible for the capacity loss during cycling, could be related to passive layer formation or structural changes on the  during the charge/discharge cycling of the battery.27 The spectra of the discharging process showed
during the charge/discharge cycling of the battery.27 The spectra of the discharging process showed  and
and  with similar values and invariability, but
with similar values and invariability, but  was too high to be measured with accuracy.
was too high to be measured with accuracy.
Stability against aluminum corrosion
It is well known that the LiTFSI salt, a good lithium-salt alternative to  , causes severe corrosion to the Al current collector commonly used in lithium batteries at potentials of
, causes severe corrosion to the Al current collector commonly used in lithium batteries at potentials of  vs
vs  .28 In order to investigate this behavior in ADN-based electrolytes, an electrochemical cell was assembled using an Al wire as working electrode and an Ag wire as a reference and counter electrodes. The cell showed an open-circuit potential (OCP) of
.28 In order to investigate this behavior in ADN-based electrolytes, an electrochemical cell was assembled using an Al wire as working electrode and an Ag wire as a reference and counter electrodes. The cell showed an open-circuit potential (OCP) of  vs
vs  and
and  vs
vs  for ADN and EC:AND electrolytes, respectively. These values are much more positive than what was reported for LiTFSI electrolyte solutions in EC:DMC (
for ADN and EC:AND electrolytes, respectively. These values are much more positive than what was reported for LiTFSI electrolyte solutions in EC:DMC ( vs
vs  ) or an ionic liquid (
) or an ionic liquid ( vs
vs  ),29 indicating a better ability of ADN to passivate the Al surface, which was corroborated by the fact that the OCP became even more positive, reaching
),29 indicating a better ability of ADN to passivate the Al surface, which was corroborated by the fact that the OCP became even more positive, reaching  vs
vs  for ADN and
for ADN and  vs
vs  for EC:ADN in
for EC:ADN in  .
.
Figure 7 shows the CV recorded for ADN and 1:1 EC:ADN electrolytes where it can be seen that during the first cycle the two electrolytes showed a similar behavior that is different from the typical hysteresis loop characteristic of a pitting corrosion. Instead, the electrolytes showed a gradual increase in the current in the forward scan that decreased in the reverse scan to lower values indicative of the formation of a protective layer that might have formed from any combination of  and
and  ions depositing on the surface29 or its passivation by the nitrile groups through the lone pair electrons on the nitrogen atom, similar to what is observed in other nitriles like acetonitrile/LiTFSI electrolyte.22 Moreover, by the fifth cycle the two electrolytes show an excellent passivating behavior with currents dropping to very insignificant values above 4.3 and
ions depositing on the surface29 or its passivation by the nitrile groups through the lone pair electrons on the nitrogen atom, similar to what is observed in other nitriles like acetonitrile/LiTFSI electrolyte.22 Moreover, by the fifth cycle the two electrolytes show an excellent passivating behavior with currents dropping to very insignificant values above 4.3 and  vs
vs  for ADN and EC:ADN, respectively.
for ADN and EC:ADN, respectively.
Figure 7. CV of an Al wire in  LiTFSI electrolyte solutions of ADN and EC:ADN (1:1 by volume).
LiTFSI electrolyte solutions of ADN and EC:ADN (1:1 by volume).
General Remarks on the Toxicity of Nitriles When Used as Solvents
The toxicity of nitrile-containing compounds lies in their ease of releasing the highly toxic  cyanide ion that can happen easily during the hydrolysis of inorganic compounds like metal cyanides (e.g., HCN, KCN).21 However, this does not take place in organic nitriles because their hydrolysis (under certain conditions) leads to the formation of the corresponding carboxelates or aldehydes, and their reduction product is most likely their corresponding amines.30 This reflects in the
cyanide ion that can happen easily during the hydrolysis of inorganic compounds like metal cyanides (e.g., HCN, KCN).21 However, this does not take place in organic nitriles because their hydrolysis (under certain conditions) leads to the formation of the corresponding carboxelates or aldehydes, and their reduction product is most likely their corresponding amines.30 This reflects in the  value of ADN, which is
value of ADN, which is  that is 31 times less toxic than KCN
that is 31 times less toxic than KCN  . However, reductive decyanation can take place in the presence of very aggressive reagents like
. However, reductive decyanation can take place in the presence of very aggressive reagents like  , or alkali metals, but in most cases the released cyanide anion is involved in further reactions. This is evident in the case of a
, or alkali metals, but in most cases the released cyanide anion is involved in further reactions. This is evident in the case of a  battery, where acetonitrile is used along with LiBr as an electrolyte, and despite the passivating film that is formed on lithium metal by the reduction of
battery, where acetonitrile is used along with LiBr as an electrolyte, and despite the passivating film that is formed on lithium metal by the reduction of  protecting acetonitrile from the metal surface, the formation of a nonpolymeric, organic nitrile-containing compound was suggested.31
protecting acetonitrile from the metal surface, the formation of a nonpolymeric, organic nitrile-containing compound was suggested.31
Conclusions
Lithium electrolytes based on adiponitrile were prepared and tested in Li-ion batteries. Adiponitrile was chosen for its high anodic and thermal stability. Its use as a single solvent in the batteries using graphite as an anode produced poor performance, and therefore, the effects of additions of a cosolvent and a cosalt were evaluated. Quite good capacity and cycling behavior was achieved with a  LiTFSI,
LiTFSI,  LiBOB, EC:ADN (1:1) electrolyte composition in a cell incorporating a graphite anode and a
LiBOB, EC:ADN (1:1) electrolyte composition in a cell incorporating a graphite anode and a  cathode. This opens the door to the assembly of safer batteries with conventional electrodes or higher energy/power batteries with cathodes with higher potentials and/or capacity.
cathode. This opens the door to the assembly of safer batteries with conventional electrodes or higher energy/power batteries with cathodes with higher potentials and/or capacity.
Acknowledgments
The authors thank the co-op undergraduate students V. Ng, S. Vanderlip, and M. Wong for their help in the experimental part of the work.
National Research Council of Canada assisted in meeting the publication costs of this article.