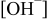Abstract
Electroless Co-based capping layers with dimethylamine borane (DMAB) and hypophosphite as reductants on Cu lines were achieved without Pd activation. The oxidation process of electroless Co-based deposition was characterized by cyclic voltammetry and electrochemical impedance spectroscopy. The oxidation of DMAB was greatly suppressed with the addition of hypophosphite during electroless CoBP deposition. At low  , an obvious oxidation peak of hypophosphite was attained due to insufficient formation of
, an obvious oxidation peak of hypophosphite was attained due to insufficient formation of  , which showed slow charge transfer on the Cu surface; at high
, which showed slow charge transfer on the Cu surface; at high  , distinct competition between hypophosphite and
, distinct competition between hypophosphite and  and sufficient
and sufficient  formed enhanced the oxidation of DMAB, indicating fast charge transfer. The selectivity of electroless Co-based deposition was also strongly influenced by [DMAB], [hypophosphite], and
formed enhanced the oxidation of DMAB, indicating fast charge transfer. The selectivity of electroless Co-based deposition was also strongly influenced by [DMAB], [hypophosphite], and  .
.
Export citation and abstract BibTeX RIS
The continuous dimension shrinkage in ultralarge-scale integration (ULSI) leads to the adoption of Cu in advanced interconnects and subsequent challenge as to the prevention of Cu diffusion into dielectric material.1 Recently, electroless metal deposition due to its inherent with simple operation condition, low processing temperature, and conformal film formation has attracted a lot of attention as potential barrier/capping layer for future USLI.2 In this respect, electroless Co films containing hypophosphite as reductant have been demonstrated to be a good barrier for Cu interconnects.3, 4 Recent applications of electroless CoB5, 6 and CoBP films6, 7 have been developed to selectively encapsulate Cu lines without conventional Pd activation. In our previous work,7 we have shown that Pd activation could induce a 8.6% increase in sheet resistance  and severe selectivity loss as compared with Pd-free process.
and severe selectivity loss as compared with Pd-free process.
It is obvious that the accomplishment of highly selective electroless Co caps on patterned structures with high aspect ratio is not easy. Therefore, the investigation of the basic mechanism and processes involved in the formation of capping layers is thus necessary for achieving selective Co deposition. Work reported by Sverdlov et al.2 focused on the employment of mixed potential theory to predict the deposition rate of electroless CoWB deposition based on many factors including concentration of the reductants, complexing agents, buffering agents, metal ion, pH, and temperature. However, thus far there have been few reports on the electrochemical study of electroless Co deposition with dimethylamine borane (DMAB) which can provide a Pd-free Cu capping process.
In this paper, our aim is to study the mechanism of Pd-free electroless CoB and CoBP systems by electrochemical techniques such as cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The effect of [DMAB], [hypophosphite], and  on the selectivity of Co deposition between Cu and dielectric surfaces was also investigated. Hopefully, the information collected could lead to improved control of electroless Co deposition without Pd activation.
on the selectivity of Co deposition between Cu and dielectric surfaces was also investigated. Hopefully, the information collected could lead to improved control of electroless Co deposition without Pd activation.
Experimental
Electrochemical investigation
All electrochemical experiments were performed in a three-electrode cell connected to a potentiostat (model 1286, Schlumberger). Pt sheet,  of Cu electrode, and saturated calomel electrode (SCE) were used as counter electrode, cathode, and reference electrode, respectively. Analytical reagent-grade chemicals and deionized (DI) water were used for electroless bath preparation and their compositions are listed in Table I.
of Cu electrode, and saturated calomel electrode (SCE) were used as counter electrode, cathode, and reference electrode, respectively. Analytical reagent-grade chemicals and deionized (DI) water were used for electroless bath preparation and their compositions are listed in Table I.
Table I. Chemicals and operating condition for electroless Co-based deposition.
| Chemicals | Function | CoB bath (M) | CoBP bath (M) |
|---|---|---|---|
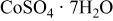
| Co source | 0.05 | 0.05 |
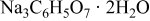
| Complexing agent | 0.4 | 0.4 |
 (DMAB) (DMAB) | Reductant | 0.01–0.1 | 0.01–0.1 |
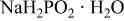
| Reductant | 0–0.2 | |
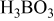
| Buffer agent | 0.2 | 0.2 |
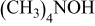 (TMAH) (TMAH) | pH adjusting agent | 0–0.112 | 0–0.112 |
| pH | 6.5–9.5 | ||
| Temperature |
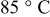
|
Anodic polarization measurements scanned from  vs SCE were performed in solutions without
vs SCE were performed in solutions without  on Cu or Co working electrode which was prepared by electroless CoB on Cu electrode. Open-circuit potential (OCP) measurements were conducted for
on Cu or Co working electrode which was prepared by electroless CoB on Cu electrode. Open-circuit potential (OCP) measurements were conducted for  without applied voltage. As for CV (cyclic voltammetry) experiments, the Cu electrode was first cathodically scanned from
without applied voltage. As for CV (cyclic voltammetry) experiments, the Cu electrode was first cathodically scanned from  vs SCE and then anodically scanned back to the starting potential. The scanned rate was
vs SCE and then anodically scanned back to the starting potential. The scanned rate was  . EIS was performed using a potentiostat with a model 1255 Schlumberger frequency analyzer. The impedance data covered a frequency range of
. EIS was performed using a potentiostat with a model 1255 Schlumberger frequency analyzer. The impedance data covered a frequency range of  . A sinusoidal ac voltage signal varying by
. A sinusoidal ac voltage signal varying by  was applied in all cases.
was applied in all cases.
Selectivity study
Electroless Co-based films on post chemical mechanical planarization (CMP) patterned Cu 
 substrate were used for the selectivity study. First, the substrate was dipped in 1%
substrate were used for the selectivity study. First, the substrate was dipped in 1%  for
for  to remove Cu oxide and then rinsed in DI water. After cleaning, the substrates were immersed into electroless baths of which the constituents and operating conditions are previously listed in Table II. The selectivity of electroless Co-based films that were deposited on Cu lines for
to remove Cu oxide and then rinsed in DI water. After cleaning, the substrates were immersed into electroless baths of which the constituents and operating conditions are previously listed in Table II. The selectivity of electroless Co-based films that were deposited on Cu lines for  was examined using field-emission scanning electron microscopy (FE-SEM, model JSM-6330F, JEOL). The deposition rate of electroless Co was investigated by a highly precise surface profilometer (model ET400a, Kosaka).
was examined using field-emission scanning electron microscopy (FE-SEM, model JSM-6330F, JEOL). The deposition rate of electroless Co was investigated by a highly precise surface profilometer (model ET400a, Kosaka).
Table II.
 and deposition rate data collected from Fig. 6 and 7, respectively.
and deposition rate data collected from Fig. 6 and 7, respectively.
| [Hypophosphite] (M) |
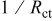
| Deposition rate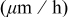
| [DMAB] (M) |
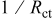
| Deposition rate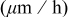
|
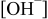 (M) (M) |
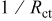
| Deposition rate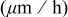
|
|---|---|---|---|---|---|---|---|---|
| 0.2 | 0.0167 | 0.4608 | 0.01 | 0.0019 | 0.6070 | 0 | 0.0334 | 0.5376 |
| 0.05 | 0.0431 | 0.8688 | 0.05 | 0.0145 | 0.8745 | 0.0175 | 0.0342 | 0.6756 |
| 0.025 | 0.0782 | 1.3110 | 0.075 | 0.0151 | 1.0887 | 0.042 | 0.0526 | 0.7440 |
| 0 | 0.1411 | 3.0315 | 0.1 | 0.0258 | 1.8167 | 0.077 | 0.0593 | 0.8856 |
| 0.112 | 0.0653 | 1.1508 |
Results and Discussion
Deposition chemistry of a Pd-free electroless Co cap on the Cu surface
A summary of the possible reactions involved with DMAB or DMAB/hypophosphite as reductant is as follows2, 8–10
1. Anodic reaction:
DMAB with sufficient  primarily proceeds to generate the reactive intermediate
primarily proceeds to generate the reactive intermediate  , which continues to undergo multielectron transfer until
, which continues to undergo multielectron transfer until  is formed.10 It can be presented as
is formed.10 It can be presented as
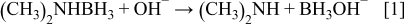
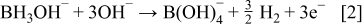
As for the DMAB/hypophosphite system, the electrons are also generated from hypophosphite and  according to the following reaction
according to the following reaction
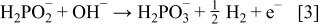
2. Cathodic reaction:
There are four cathodic reactions involved including (i) reduction of  ; (ii) reduction of
; (ii) reduction of  ; (iii) reduction of B; and (iv) reduction of P. The electrons generated from Eq. 1, 2, 3 are mostly consumed for the reduction of
; (iii) reduction of B; and (iv) reduction of P. The electrons generated from Eq. 1, 2, 3 are mostly consumed for the reduction of 
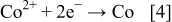
Part of the electrons is used for the reduction of  that leads to hydrogen evolution.
that leads to hydrogen evolution.
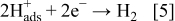
Codeposition of elemental P and B also takes place according to the following reactions
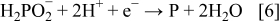
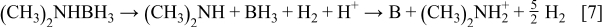
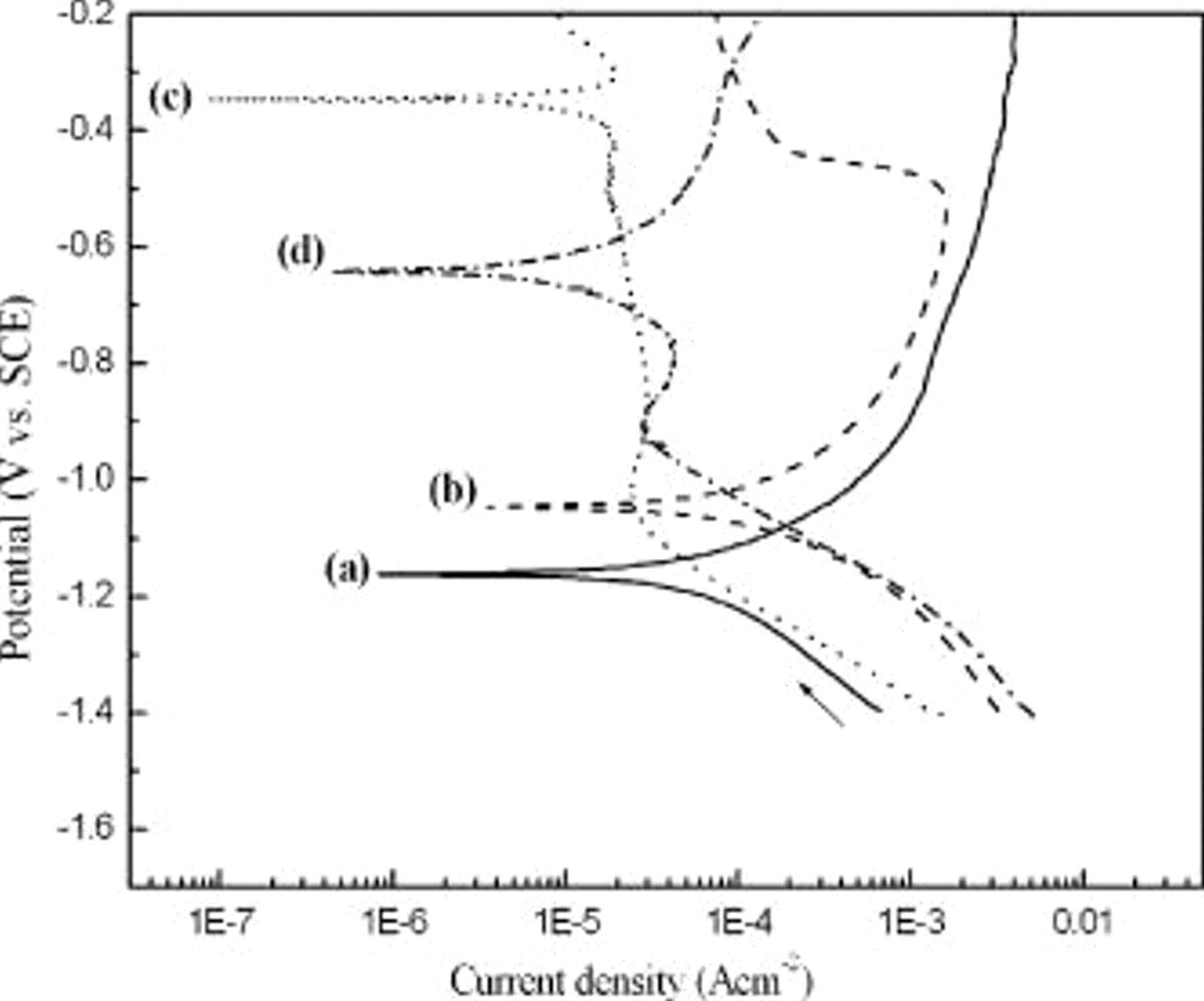
Anodic polarization measurements shown in Fig. 1 were carried out to investigate the oxidation of DMAB and hypophosphite on Cu or Co electrode. As for the oxidation of DMAB, the Cu electrode potential was approximately  vs SCE, which is slightly more negative than Co electrode potential (
vs SCE, which is slightly more negative than Co electrode potential ( vs SCE). However, the distinctly more positive Cu electrode potential appeared at
vs SCE). However, the distinctly more positive Cu electrode potential appeared at  vs SCE rather than Co (
vs SCE rather than Co ( vs SCE) in hypophosphite only solution. The order of anodic current of DMAB and hypophosphite on Cu and Co electrode is: DMAB on Cu
vs SCE) in hypophosphite only solution. The order of anodic current of DMAB and hypophosphite on Cu and Co electrode is: DMAB on Cu  on Co
on Co  on Co
on Co  on Cu electrode. According to the above results, Cu shows excellently high catalytic activity for the oxidation of DMAB but poor catalytic activity for the oxidation of hypophosphite.
on Cu electrode. According to the above results, Cu shows excellently high catalytic activity for the oxidation of DMAB but poor catalytic activity for the oxidation of hypophosphite.
Figure 1. Anodic polarization curves of reductants including (a) DMAB  on Cu electrode, (b) DMAB
on Cu electrode, (b) DMAB  on Co electrode, (c) hypophosphite
on Co electrode, (c) hypophosphite  on Cu electrode, and (d) hypophosphite
on Cu electrode, and (d) hypophosphite  on Co electrode.
on Co electrode.
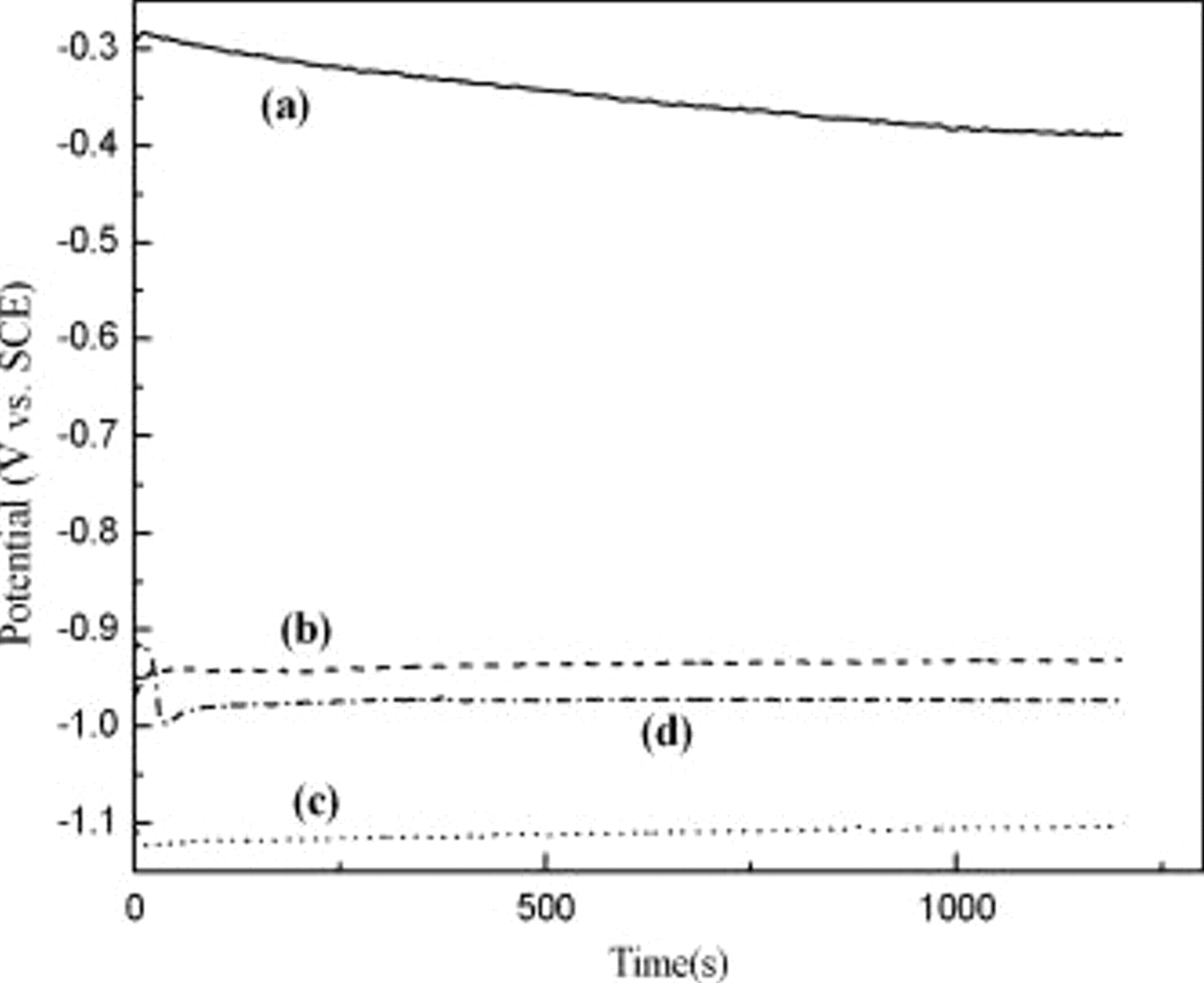
Figure 2 depicts the OCP of electroless Co solutions with hypophosphite, DMAB, or DMAB/hypophosphite as reductant. The OCP value was about  vs SCE with CoP bath in which no Co deposit could be found on Cu surface. This confirms again the fact that the Cu surface is not catalytically active for oxidation of hypophosphite, which is also supported by Homma's theoretical calculation.11 The OCP value was driven very negative, about
vs SCE with CoP bath in which no Co deposit could be found on Cu surface. This confirms again the fact that the Cu surface is not catalytically active for oxidation of hypophosphite, which is also supported by Homma's theoretical calculation.11 The OCP value was driven very negative, about  ,
,  vs SCE for CoB, CoBP solutions, respectively, which consequently leads to the formation of Co deposition. The presence of hypophosphite in the CoBP shifted the OCP value to
vs SCE for CoB, CoBP solutions, respectively, which consequently leads to the formation of Co deposition. The presence of hypophosphite in the CoBP shifted the OCP value to  vs SCE, which is slightly more positive than in CoB solution. It thus appears that DMAB has an enhancing effect on the catalytic initiation of Co deposition on Cu so that Pd activation can be waived.
vs SCE, which is slightly more positive than in CoB solution. It thus appears that DMAB has an enhancing effect on the catalytic initiation of Co deposition on Cu so that Pd activation can be waived.
Figure 2. OCP of Cu electrode in the electroless Co-based solution including (a) Cu in CoP solution, (b)  in CoP solution, (c) Cu in CoB solution, and (d) Cu in CoBP solution (
in CoP solution, (c) Cu in CoB solution, and (d) Cu in CoBP solution ( , [hypophosphite], [DMAB]:
, [hypophosphite], [DMAB]:  ).
).
Study of the deposition process by CV scans
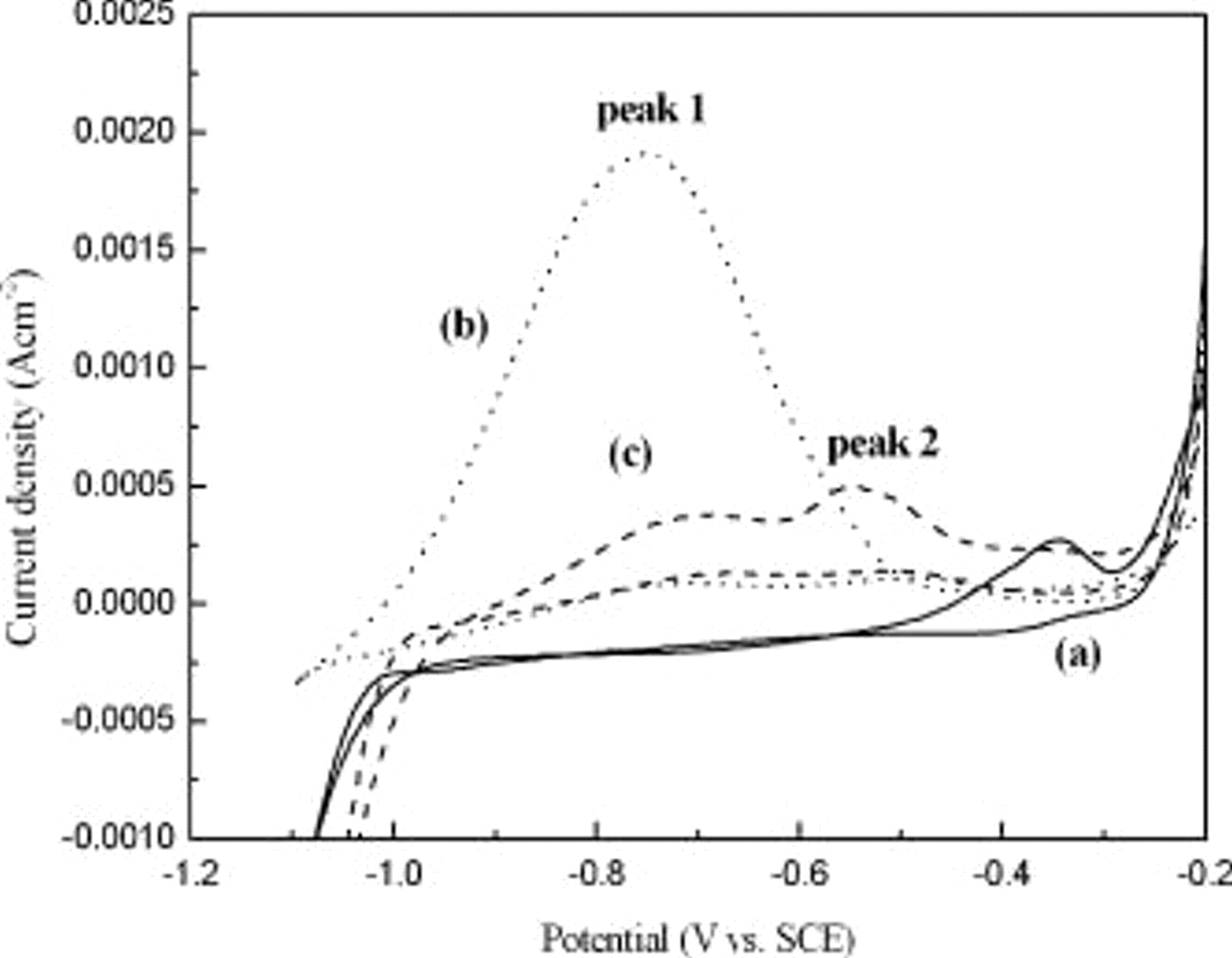
CV was employed to identify the redox reactions occurring in the various solutions to evaluate the effect of the basic electrolyte constituents on the redox reactions. Figure 3 shows the cyclic voltammograms in the range between  and
and  vs SCE. In Co solution without redutants, no oxidation peak of reductants but one oxidation peak of Cu was observed, as shown in Fig. 3a. By contrast, curve (b) contains DMAB and peak 1, a large and broad oxidation peak of
vs SCE. In Co solution without redutants, no oxidation peak of reductants but one oxidation peak of Cu was observed, as shown in Fig. 3a. By contrast, curve (b) contains DMAB and peak 1, a large and broad oxidation peak of  on the Cu surface which is considered a reactive intermediate generated from DMAB. The formation of a "true" reducing agent,
on the Cu surface which is considered a reactive intermediate generated from DMAB. The formation of a "true" reducing agent,  , was reported to undergo oxidation at
, was reported to undergo oxidation at  vs SCE.12–15 The oxidation process of DMAB shows irreversible behavior. Figure 3c shows the cyclic voltammogram of CoBP solution. This anodic scan reveals the oxidation of both
vs SCE.12–15 The oxidation process of DMAB shows irreversible behavior. Figure 3c shows the cyclic voltammogram of CoBP solution. This anodic scan reveals the oxidation of both  and hypophosphite, which are ascribed to peak 1 and peak 2 (
and hypophosphite, which are ascribed to peak 1 and peak 2 ( vs SCE), respectively. The intensity of peak 1 greatly decreases from
vs SCE), respectively. The intensity of peak 1 greatly decreases from  upon the addition of hypophosphite, indicating that hypophosphite may suppress the oxidation of
upon the addition of hypophosphite, indicating that hypophosphite may suppress the oxidation of  . Some researchers believe that the action of hypophosphite may lower the hydrogen overvoltage and thus prevent the formation of metal reduction.16 In other words, hypophosphite may strongly adsorb or dehydrogenate on the Cu surface, thereby inhibiting the presence of DMAB on the surface.
. Some researchers believe that the action of hypophosphite may lower the hydrogen overvoltage and thus prevent the formation of metal reduction.16 In other words, hypophosphite may strongly adsorb or dehydrogenate on the Cu surface, thereby inhibiting the presence of DMAB on the surface.
Figure 3. CV analysis of Co-based solution with (a) Co solution, (b) CoB solution, and (c) CoBP solution ( , [hypophosphite], [DMAB]:
, [hypophosphite], [DMAB]:  ).
).
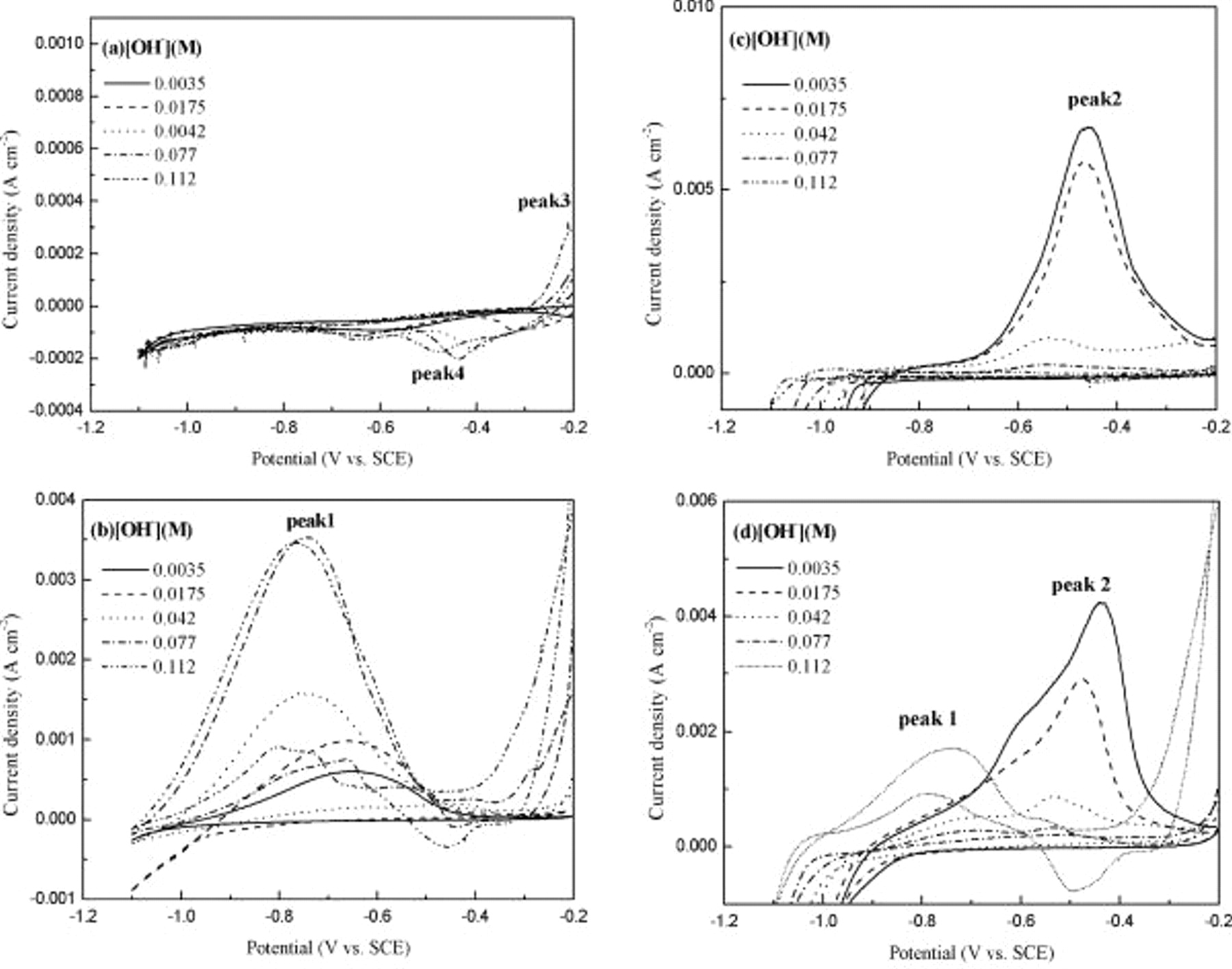
Figure 4 shows the cyclic voltammograms ranging between  and
and  vs SCE in Co-based solutions with
vs SCE in Co-based solutions with  varying from
varying from  . A cyclic voltammogram of Co solution without reductants and
. A cyclic voltammogram of Co solution without reductants and  only is shown in Fig. 4a. Peak 3, the anodic peak at
only is shown in Fig. 4a. Peak 3, the anodic peak at  vs SCE, indicates that surface Cu atoms interact with
vs SCE, indicates that surface Cu atoms interact with  in solution to form a passivated Cu oxide and passivation increases with
in solution to form a passivated Cu oxide and passivation increases with  . The cathodic scan shows the reduction peaks (peak 4) for Cu oxide from
. The cathodic scan shows the reduction peaks (peak 4) for Cu oxide from  vs SCE. Figure 4b shows a change in oxidation behavior of DMAB with varying
vs SCE. Figure 4b shows a change in oxidation behavior of DMAB with varying  when DMAB was added into the Co solution at
when DMAB was added into the Co solution at  . In general, peak 1, the reactivation peak, increases in height and area with an increase in
. In general, peak 1, the reactivation peak, increases in height and area with an increase in  , suggesting that high
, suggesting that high  is favorable for DMAB oxidation on the Cu surface. According to Eq. 1, 2, the amount of
is favorable for DMAB oxidation on the Cu surface. According to Eq. 1, 2, the amount of  intermediate generated and continued oxidation of
intermediate generated and continued oxidation of  depend on the
depend on the  of the solution. Additionally, peak 1 shifts cathodically with increasing
of the solution. Additionally, peak 1 shifts cathodically with increasing  . As the scan is reversed, appreciable anodic peak current flows and a corresponding reduction peak is absent, indicating the irreversible nature of
. As the scan is reversed, appreciable anodic peak current flows and a corresponding reduction peak is absent, indicating the irreversible nature of  oxidation. The oxidation behavior of hypophosphite
oxidation. The oxidation behavior of hypophosphite  on Cu is shown in Fig. 4c. The intensity of peak 2 greatly decreases with increasing
on Cu is shown in Fig. 4c. The intensity of peak 2 greatly decreases with increasing  , meaning the oxidation of these two reductants on the Cu surface shows totally different responses with respect to
, meaning the oxidation of these two reductants on the Cu surface shows totally different responses with respect to  . This is probably because the adsorption competition between hypophosphite and
. This is probably because the adsorption competition between hypophosphite and  occurs, leading to decreased surface concentration of hypophosphite, thereby inhibiting the oxidation of hypophosphite. The effect of
occurs, leading to decreased surface concentration of hypophosphite, thereby inhibiting the oxidation of hypophosphite. The effect of  on the oxidation of both reductants in CoBP solution is presented in Fig. 4d. At low
on the oxidation of both reductants in CoBP solution is presented in Fig. 4d. At low  , the oxidation peak of hypophosphite (peak 2) is dominant in the CoBP solution, because insufficient
, the oxidation peak of hypophosphite (peak 2) is dominant in the CoBP solution, because insufficient  is available for DMAB to generate the reactive species,
is available for DMAB to generate the reactive species,  , and thus the oxidation peak of
, and thus the oxidation peak of  become less obvious. However, at high
become less obvious. However, at high  of
of  , competition between hypophosphite and
, competition between hypophosphite and  favors the formation of
favors the formation of  , resulting in a distinct oxidation peak of
, resulting in a distinct oxidation peak of  (peak 1). These results suggest that the oxidation process in CoBP solution shows strong
(peak 1). These results suggest that the oxidation process in CoBP solution shows strong  dependence that may influence the nucleation and growth behavior of a Pd-free electroless Co layer on Cu surface.
dependence that may influence the nucleation and growth behavior of a Pd-free electroless Co layer on Cu surface.
Figure 4. CV investigations in (a) Co solution without reductants, (b) CoB solution, (c) CoP solution, and (d) CoBP solution with 
 (
( , [hypophosphite], [DMAB]:
, [hypophosphite], [DMAB]:  ).
).
The kinetics of Pd-free electroless Co deposition on the Cu surface
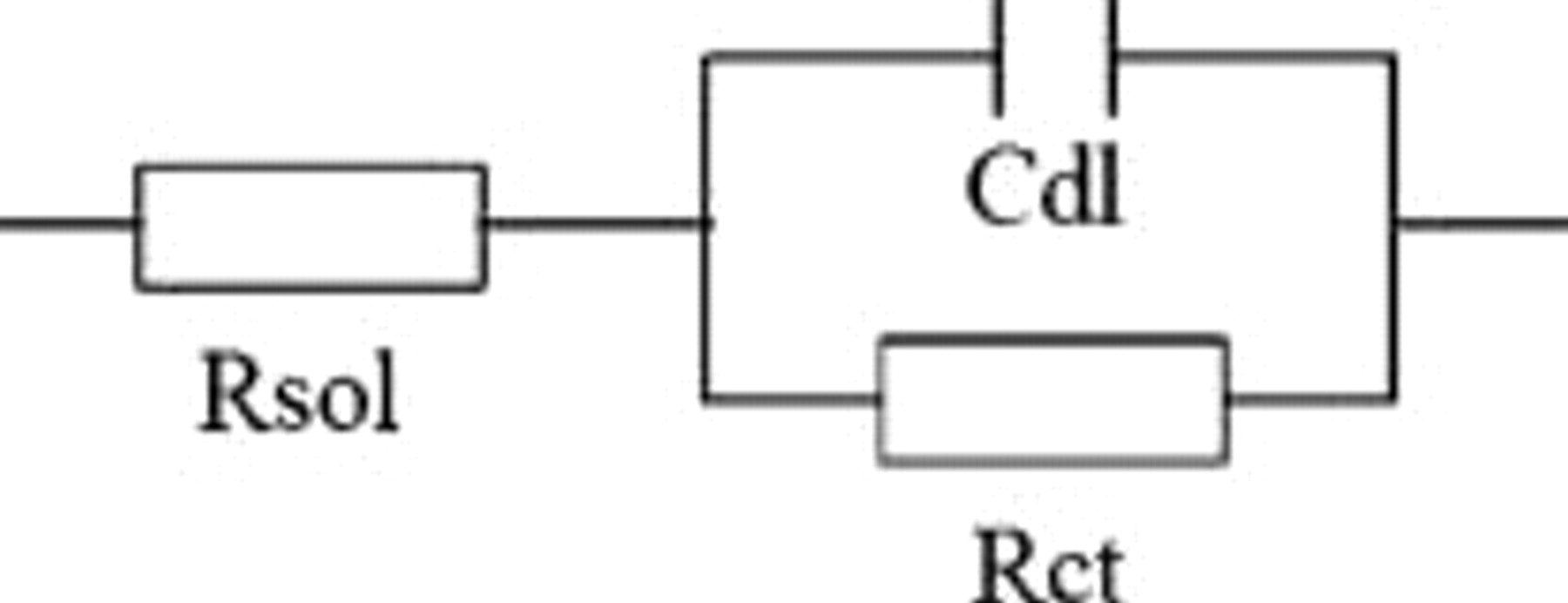
EIS was further used to gain both kinetic and mechanistic information on the interface reactions occurring during electroless Co deposition. Electroless Co systems which are entirely under charge-transfer control can be described by the simple equivalent circuit shown in Fig. 5,17 in which  is the capacitance of the electrode surface and
is the capacitance of the electrode surface and  is the net charge-transfer resistance of the electroless deposition reaction. All ohmic resistances in the system such as the electrolyte resistance and cable resistance are contained in
is the net charge-transfer resistance of the electroless deposition reaction. All ohmic resistances in the system such as the electrolyte resistance and cable resistance are contained in  , the solution resistance term.
, the solution resistance term.  should in principle be inversely proportional to the deposition rate18
should in principle be inversely proportional to the deposition rate18
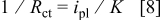
where  is the exchange current and
is the exchange current and  is the mass-transfer coefficients and is equal to
is the mass-transfer coefficients and is equal to  , where
, where  is the number of electrons transferred.
is the number of electrons transferred.  can easily be evaluated from the diameter of Nyquist impedance diagrams (
can easily be evaluated from the diameter of Nyquist impedance diagrams ( vs
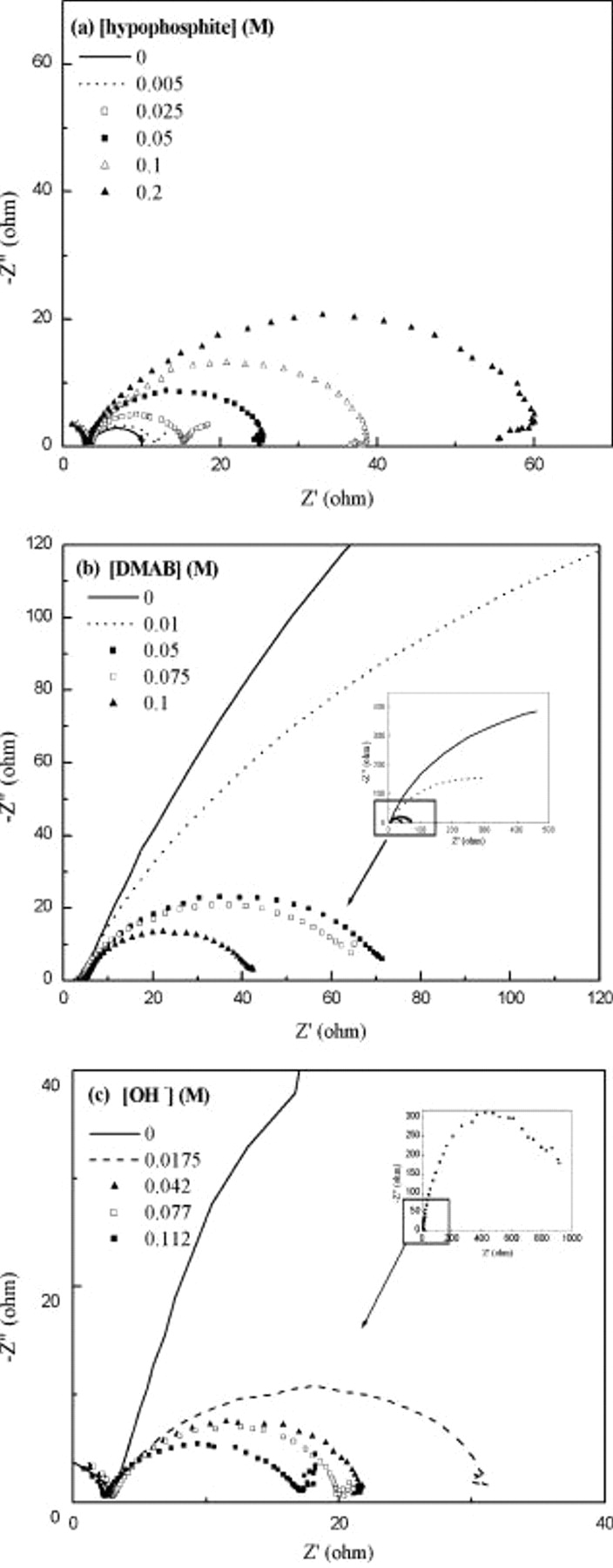
vs  ).19 Figure 6 shows Nyquist plots of CoBP solutions with varying [hypophosphite], [DMAB], and
).19 Figure 6 shows Nyquist plots of CoBP solutions with varying [hypophosphite], [DMAB], and  . Figure 6 clearly shows the expected semicircle behavior, indicating the deposition process is largely a surface-controlled reaction. The
. Figure 6 clearly shows the expected semicircle behavior, indicating the deposition process is largely a surface-controlled reaction. The  value became larger as [hypophosphite] increased, indicating that addition of hypophosphite induces a delay of charge transfer on the Cu surface, as shown in Fig. 6a. In Fig. 6b, the
value became larger as [hypophosphite] increased, indicating that addition of hypophosphite induces a delay of charge transfer on the Cu surface, as shown in Fig. 6a. In Fig. 6b, the  value ranged from
value ranged from  as [DMAB] varied from
as [DMAB] varied from  , then decreased with increasing [DMAB]. This result supports the fact that electroless Co deposition is initiated by DMAB. Figure 6c shows a Nyquist plot of CoBP solutions with varying
, then decreased with increasing [DMAB]. This result supports the fact that electroless Co deposition is initiated by DMAB. Figure 6c shows a Nyquist plot of CoBP solutions with varying  . A markedly large
. A markedly large  of around
of around  was observed at extremely low
was observed at extremely low  , indicating excessively slow charge transfer on the Cu surface. In addition, it is apparent that an increase in
, indicating excessively slow charge transfer on the Cu surface. In addition, it is apparent that an increase in  results in a lower value of
results in a lower value of  . The results are also consistent with the CV analysis, which indicates the subordinated position of DMAB oxidation at low
. The results are also consistent with the CV analysis, which indicates the subordinated position of DMAB oxidation at low  and the dominant position of DMAB at high
and the dominant position of DMAB at high  .
.
Figure 5. Equivalent circuit for a simple electroless deposition system.16
Figure 6. Nyquist impedance plots for CoBP solutions with (a) [hypophosphite]:  ,
,  , [DMAB]:
, [DMAB]:  ,
,  :
:  ; (b) [DMAB]:
; (b) [DMAB]:  ,
,  , [hypophosphite]:
, [hypophosphite]:  ,
,  :
:  ; and (c)
; and (c)  :
:  ,
,  , [hypophosphite], [DMAB]:
, [hypophosphite], [DMAB]:  .
.
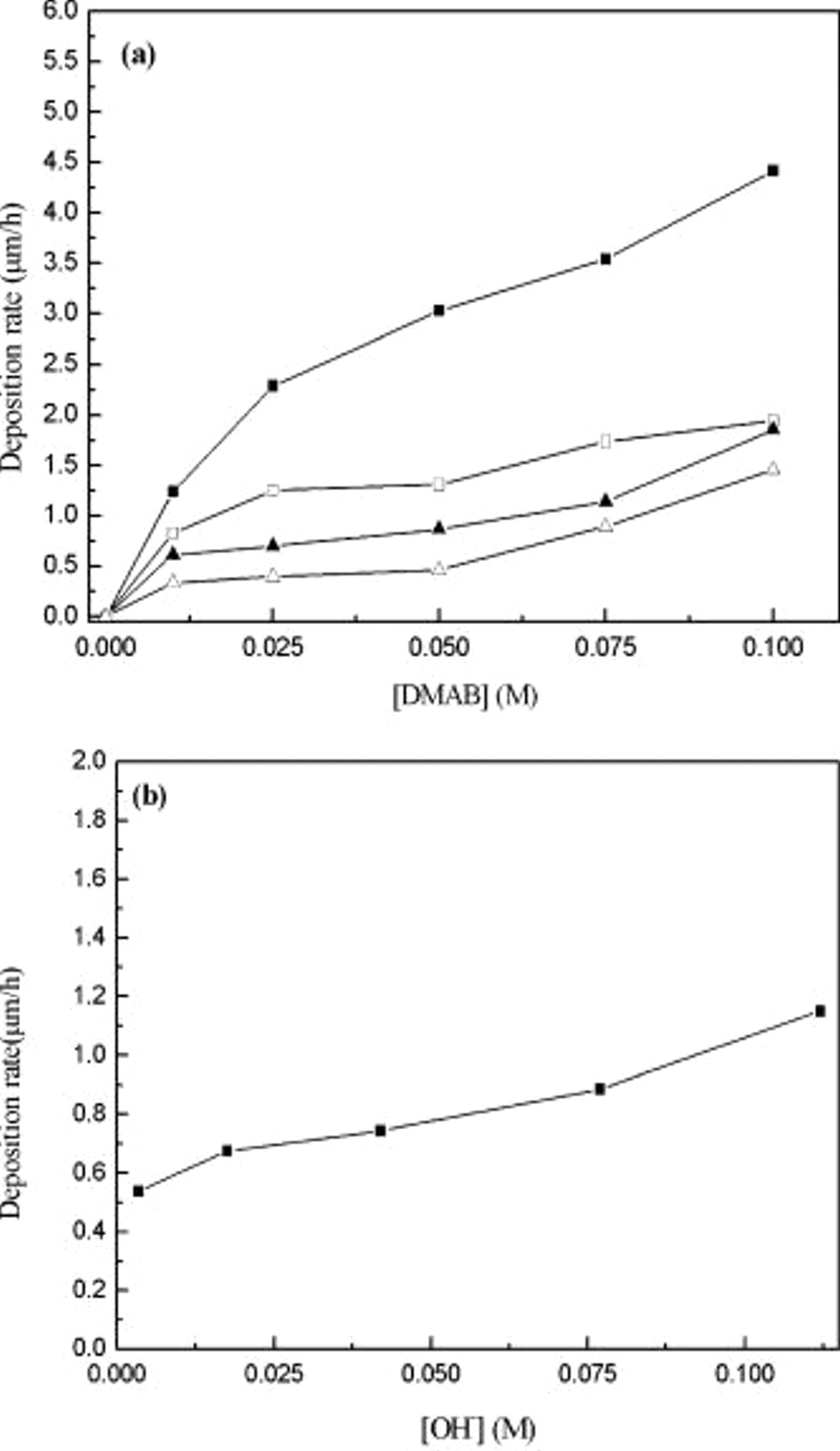
The effect of [DMAB] and [hypophosphite] on Co deposition rate was measured by a surface profilometer as shown in Fig. 7a. The deposition rate increased from  with increased [DMAB] in the CoB bath, while the deposition rate slightly increased with increasing [DMAB] in the CoBP bath. The Co deposition rate greatly decreased with increasing [hypophosphite] in the CoBP bath. For instance, at
with increased [DMAB] in the CoB bath, while the deposition rate slightly increased with increasing [DMAB] in the CoBP bath. The Co deposition rate greatly decreased with increasing [hypophosphite] in the CoBP bath. For instance, at  of [DMAB], the deposition rate was reduced from
of [DMAB], the deposition rate was reduced from  upon addition of
upon addition of  [hypophosphite]. The effect of
[hypophosphite]. The effect of  on Co deposition rate was also examined as shown in Fig. 7b. The Co deposition rate increased with an increase in
on Co deposition rate was also examined as shown in Fig. 7b. The Co deposition rate increased with an increase in  , indicating enhancement of the oxidation of reductants based on Eq. 1, 2. Table II shows a combination of
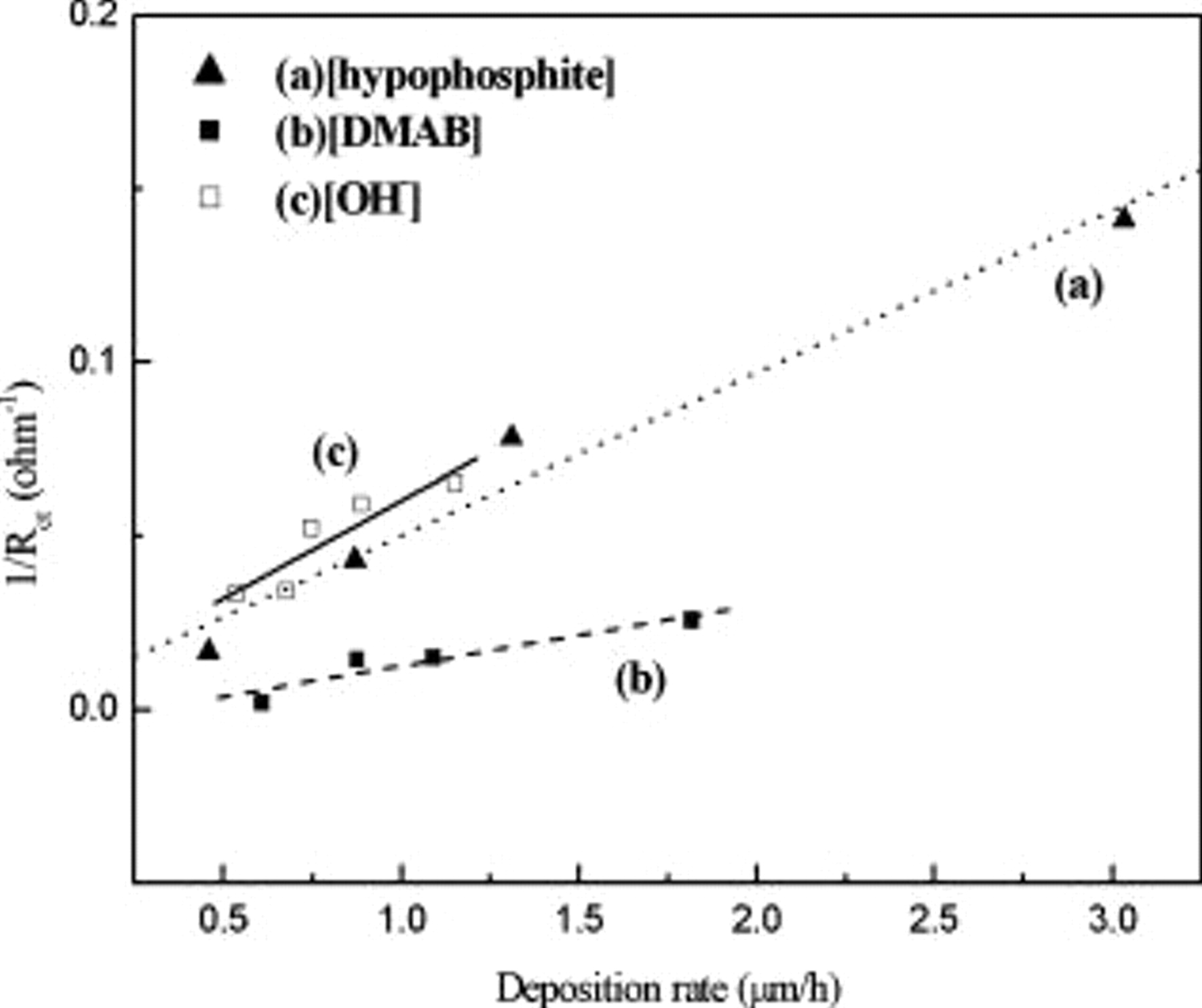
, indicating enhancement of the oxidation of reductants based on Eq. 1, 2. Table II shows a combination of  data collected form Fig. 6 and deposition rate data from Fig. 7 based on various amounts of [hypophosphite], [DMAB], and
data collected form Fig. 6 and deposition rate data from Fig. 7 based on various amounts of [hypophosphite], [DMAB], and  that can lead to a plot of
that can lead to a plot of  vs deposition rate, as depicted in Fig. 8. The predicted linear behavior in Fig. 8 also confirms the validity of Eq. 8.
vs deposition rate, as depicted in Fig. 8. The predicted linear behavior in Fig. 8 also confirms the validity of Eq. 8.
Figure 7. Relationship between electroless Co deposition rate and (a) reductants ( :
:  ,
,  :
:  , [DMAB]:
, [DMAB]:  , [hypophosphite]: (■) 0, (◻) 0.025, (▴) 0.05, and (▵)
, [hypophosphite]: (■) 0, (◻) 0.025, (▴) 0.05, and (▵)  ); (b)
); (b)  :
:  (
( , [hypophosphite], [DMAB]:
, [hypophosphite], [DMAB]:  ).
).
Figure 8.
 vs deposition rate dependence with (a) [hypophosphite], (b) [DMAB], and (c)
vs deposition rate dependence with (a) [hypophosphite], (b) [DMAB], and (c)  . (
. ( and deposition rate calculated from Fig. 6 and 7, respectively.)
and deposition rate calculated from Fig. 6 and 7, respectively.)
Selectivity control by [DMAB], [hypophosphite], and 
Electrons derived from the oxidation of reductants on the catalytic Cu surface simultaneously reduce  to Co deposit on Cu lines. According to Eq. 1, 2, 3 and our CV results,
to Co deposit on Cu lines. According to Eq. 1, 2, 3 and our CV results,  strongly controls the oxidation of DMAB and hypophosphite. Therefore, the selectivity of electroless Co deposition on Cu lines would be governed by factors such as [DMAB], [hypophosphite], and
strongly controls the oxidation of DMAB and hypophosphite. Therefore, the selectivity of electroless Co deposition on Cu lines would be governed by factors such as [DMAB], [hypophosphite], and  . First, the effect of [DMAB]
. First, the effect of [DMAB]  and [hypophosphite]
and [hypophosphite]  on the selectivity of electroless Co deposition was investigated by SEM and summarized in Table III. It can be seen that unwanted Co deposition on the dielectric region became severe with increasing [DMAB] in the CoB solution. However, the extraneous Co deposition on the dielectric surface was suppressed by increasing [hypophosphite] in CoBP solution. The effect of
on the selectivity of electroless Co deposition was investigated by SEM and summarized in Table III. It can be seen that unwanted Co deposition on the dielectric region became severe with increasing [DMAB] in the CoB solution. However, the extraneous Co deposition on the dielectric surface was suppressed by increasing [hypophosphite] in CoBP solution. The effect of  on the selectivity of electroless Co deposition was adjusted with
on the selectivity of electroless Co deposition was adjusted with  of
of  to attain pH from 6.5 to 9.5 by tetramethyl ammonium hydroxide (TMAH) as shown in Fig. 9. Electroless Co film was not formed on the Cu pattern without the addition of TMAH (pH 6.5), because the oxidation reaction of DMAB could not occur on the Cu surface, necessary for the initiation of electroless Co deposition. Apparently, electroless Co deposition can be obtained on the Cu surface at above
to attain pH from 6.5 to 9.5 by tetramethyl ammonium hydroxide (TMAH) as shown in Fig. 9. Electroless Co film was not formed on the Cu pattern without the addition of TMAH (pH 6.5), because the oxidation reaction of DMAB could not occur on the Cu surface, necessary for the initiation of electroless Co deposition. Apparently, electroless Co deposition can be obtained on the Cu surface at above 
 . However, extraneous Co deposition on the dielectric also increased by increasing
. However, extraneous Co deposition on the dielectric also increased by increasing  over
over  . So highly selective Co deposition on Cu lines can be achieved by controlling [hypophosphite] (between 0.025 and
. So highly selective Co deposition on Cu lines can be achieved by controlling [hypophosphite] (between 0.025 and  ), [DMAB] (between 0.025 and
), [DMAB] (between 0.025 and  ), and
), and  (between
(between  and
and  ).
).
Table III. Effects of [DMAB] and [hypophosphite] on the selectivity of electroless Co-based deposition.
| B | A | |||
|---|---|---|---|---|
| 0 | 0.025 | 0.05 | 0.2 | |
| 0.01 | ◻ | ○ | ○ | × |
| 0.025 | ◻ | ○ | ○ | ○ |
| 0.05 | ◻ | ◻ | ○ | ○ |
| 0.075 | ◻ | ◻ | ◻ | ◻ |
| 0.1 | ◻ | ◻ | ◻ | ◻ |
a(A) hypophosphite concentration (M), (B) DMAB concentration (M), (○) selective Co deposition on Cu lines, (◻) extraneous Co deposition on dielectric surface, and (×) no Co deposition.
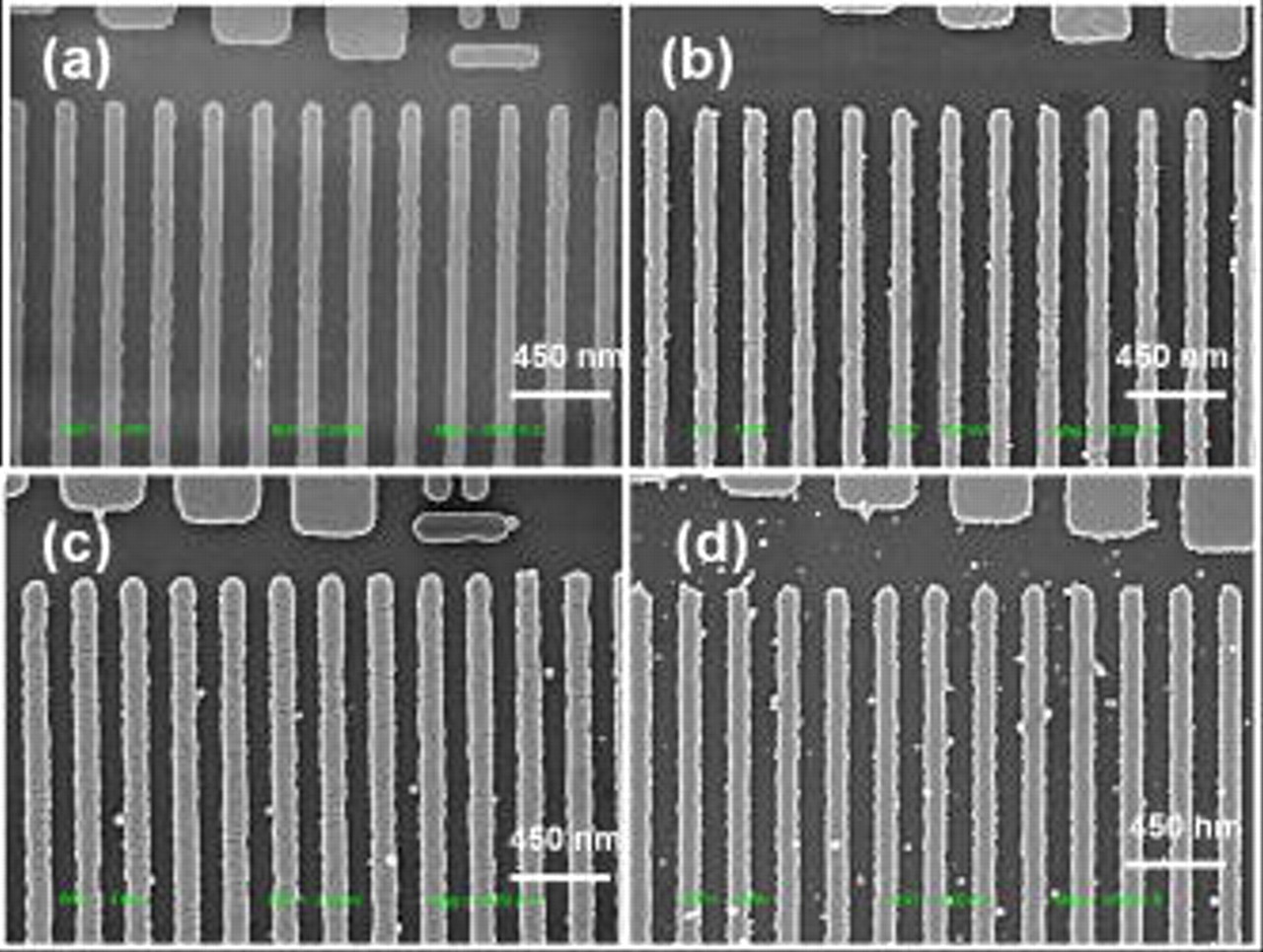
Figure 9. (Color online) SEM images of selectivity study of electroless Co depositions on Cu lines with varying  : (a) 0, (b) 0.0175, (c) 0.077, and (d)
: (a) 0, (b) 0.0175, (c) 0.077, and (d)  .
.
Conclusion
Because the formation of Pd-free electroless Co films cannot be initiated on Cu surfaces with hypophosphite as the reductant, the initiation of Pd-free electroless Co deposition was carried out by using DMAB as a reductant in the basic media on catalytic Cu surfaces. The exposed Cu surface was first covered by a Co layer that could provide a catalytically active surface toward the oxidation of hypophosphite and DMAB. The oxidation of DMAB was inhibited by the hypophosphite in electroless CoBP bath. Additionally, the oxidation of DMAB and hypophosphite during electroless CoBP deposition showed strong  dependence. Therefore, for depositing highly selective electroless CoBP films, the recommended operating conditions are as follows: [hypophosphite]
dependence. Therefore, for depositing highly selective electroless CoBP films, the recommended operating conditions are as follows: [hypophosphite]  , [DMAB]
, [DMAB]  , and
, and 
 .
.
Acknowledgments
This work was partially supported by the National Science Council of Taiwan (contract no. NSC-95-2221-E-007-206) and Tsing Hua University.
National Tsing Hua University assisted in meeting the publication costs of this article.