Abstract
Gold electrodeposition on n-Si(100) surface was investigated by performing a detailed study on nucleation and growth. The effects of additives such as thallium, applied potential and current density, and bulk gold concentration were studied. With addition of thallium in the solution, enhancement and inhibition of the gold deposition were observed at low potential and high potential, respectively. The nucleation of gold on Si was found to depend on the Au concentration and applied potential as well as the  addition. At low current densities
addition. At low current densities  increases the Au nuclei size and decreases the density by catalyzing the nuclei growth. At high current densities, an opposite effect was observed. An adsorbed thallium intermediate species is proposed to explain both the catalytic and suppressive effects.
increases the Au nuclei size and decreases the density by catalyzing the nuclei growth. At high current densities, an opposite effect was observed. An adsorbed thallium intermediate species is proposed to explain both the catalytic and suppressive effects.
Export citation and abstract BibTeX RIS
Compared to metal electrodeposition on metal substrates, the electrodeposition on semiconductors is different at two aspects, the semiconductor characteristics of the substrate and the weak interaction between the semiconductor material and most metal species. In contrast to the continuous electron energy levels in metals, the electron energy levels in semiconductors form two energy bands, a valence band and conduction band, which are separated by a certain energy gap, the bandgap. Furthermore, due to the limited number of carriers available in semiconductors, the charge in the substrate is distributed across a space-charge region instead of a surface-charge layer in metal substrate. The semiconductor–electrolyte interface forms a Schottky barrier, similar to most metal-semiconductor contacts, except that the work function of the metal is substituted by the Fermi level of the redox couple in electrolyte. Therefore, at equilibrium and depletion conditions, a fluctuation in the applied potential only changes the charge distribution across the space-charge region, or the band-bending, while the potential drop across the Helmholtz layer remains constant. Only when a significant cathodic (for n-type Si) current flows from the semiconductor substrate to the electrolyte, the potential across the Helmholtz layer changes.1
In addition to the semiconducting nature of the substrate, metal electrodeposition on semiconductors is also complicated by the nucleation process. Due to the weak interaction between the metal and the semiconductor, the metal nucleation follows the Volmer–Weber mechanism, an island formation, and transport-controlled 3D growth process. This process can occur with electrodeposition on foreign substrates when the interaction between deposit and substrate is weak. The mechanism has been extensively studied and the two extreme cases, instantaneous and progressive nucleation, have been well described.2–4
In spite of the fact that metal electrodeposition on semiconductor substrates might allow the direct formation of Schottky contact and the direct integration of metal electrodeposition with other semiconductor processes, this subject has not been addressed until recently, when a few groups reported some studies on different systems.5–17 Among them, gold has been studied most widely due to its weak interaction with silicon substrate and its noble reversible potential, which differentiates from side reactions and thus eases the electrochemical analysis. Depestel and Strubbe5 studied the Au nucleation on GaAs in a cyanide-based alkaline chemistry and found that the nucleation depended strongly on the crystal orientation of GaAs substrate, probably due to the different chemical composition on the different crystal surfaces. Oskam et al. studied the Au electrodeposition on Si in a similar chemistry, where a progressive nucleation was observed.10, 18, 19 They plated a high-quality  Schottky structure with a pulse scheme, where a high potential nucleation pulse was followed by a low potential for film growth.18 More recently, the effect of iodide ion was studied on Au electrodeposition on a glassy carbon electrode. A high nucleation density and a preferred Au(111) orientation were observed.20
Schottky structure with a pulse scheme, where a high potential nucleation pulse was followed by a low potential for film growth.18 More recently, the effect of iodide ion was studied on Au electrodeposition on a glassy carbon electrode. A high nucleation density and a preferred Au(111) orientation were observed.20
For most applications of metal on semiconductor, high nucleation density and uniform nuclei size are required. One of the ways to tailor the nucleation process is the application of additives. Thallium has been known as a grain refiner in gold electrodeposition, which is believed to modify the nucleation and growth phenoma. In this report, our studies are focused on the effect of thallium addition on Au deposition on n-Si. The effects of thallium on Au bulk film electrodeposition as well as on the initial nucleation stages were investigated.
Experimental
Experiments were carried out in a traditional three-electrode cell, with a platinum mesh as counter electrode and a saturated calomel electrode (SCE) as reference. The working electrode is a rotating disk electrode (RDE) in which a Si piece was mounted. The Si wafers used were antimony-doped n-Si(100) with resistivity between 0.008 and  . The Si pieces were cleaned by a standard Huang clean process to remove organic contamination and surface oxide. A dilute hydroflouric acid dip for
. The Si pieces were cleaned by a standard Huang clean process to remove organic contamination and surface oxide. A dilute hydroflouric acid dip for  followed by ethanol rinse and nitrogen dry was carried out immediately before the Si was mounted into the RDE for electrodeposition. InGa eutectic was used to ensure the ohmic contact.
followed by ethanol rinse and nitrogen dry was carried out immediately before the Si was mounted into the RDE for electrodeposition. InGa eutectic was used to ensure the ohmic contact.
Neutronex 309 Au solution from Cookson Electronics, Inc., a sulfite-based Au chemistry, was used as the gold solution makeup. Solutions with desired Au concentration were obtained by diluting the original solution, with pH kept between 9.0 and 9.3. Reagent-grade thallium(I) acetate,  , from Aldrich, Inc., was used as an additive for Au electrodeposition. An EG&G 273 potentiostat by Princeton Applied Research, Inc., was used as a power source for most of the electrochemical studies. The nucleation studies were performed using an AutoLab PGSTAT30 by Eco Chemie LLC by applying a short cathodic pulse on a high-impedance Si substrate. All experiments in this report were carried out at room temperature.
, from Aldrich, Inc., was used as an additive for Au electrodeposition. An EG&G 273 potentiostat by Princeton Applied Research, Inc., was used as a power source for most of the electrochemical studies. The nucleation studies were performed using an AutoLab PGSTAT30 by Eco Chemie LLC by applying a short cathodic pulse on a high-impedance Si substrate. All experiments in this report were carried out at room temperature.
Results and Discussion
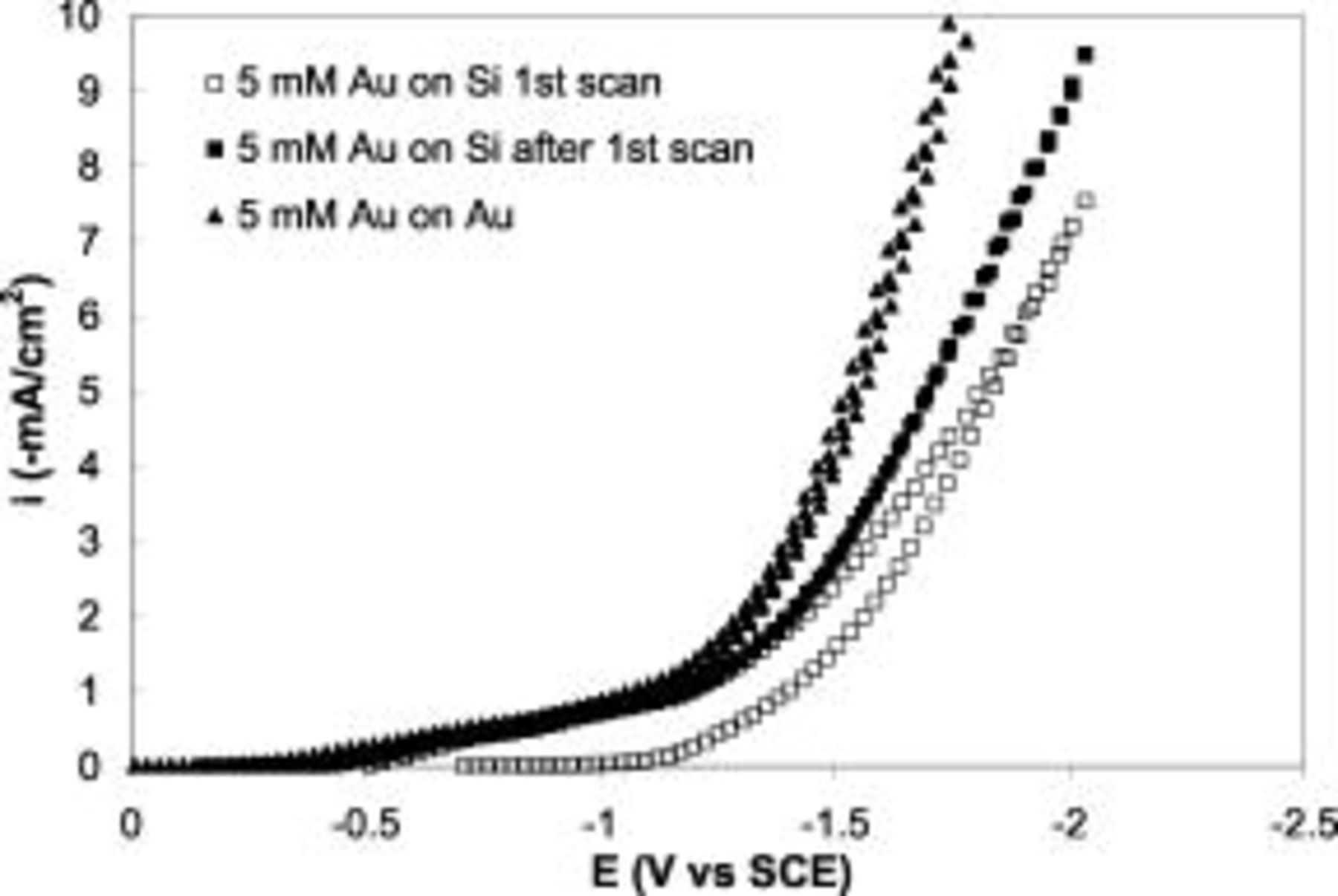
Figure 1 shows two cyclic voltammograms in a  Au electrolyte at
Au electrolyte at  on n-Si and Au disk electrodes, respectively. A scan rate of
on n-Si and Au disk electrodes, respectively. A scan rate of  was used to avoid surface area deviation due to significant Au deposition. No underpotential deposition was observed for Au electrodeposition on Si due to the weak interaction between Au and Si. On the contrary, a nucleation overpotential of
was used to avoid surface area deviation due to significant Au deposition. No underpotential deposition was observed for Au electrodeposition on Si due to the weak interaction between Au and Si. On the contrary, a nucleation overpotential of  was observed, suggesting that Au electrodeposition occurs preferentially on Au over Si. After the Si surface was completely covered with the plated Au after first scan, the plating became identical to Au electrode at low potential. At high potential, the electrodeposition current on Si was significantly lower than the Au electrode due to the higher resistivity of the Si substrate.
was observed, suggesting that Au electrodeposition occurs preferentially on Au over Si. After the Si surface was completely covered with the plated Au after first scan, the plating became identical to Au electrode at low potential. At high potential, the electrodeposition current on Si was significantly lower than the Au electrode due to the higher resistivity of the Si substrate.
Figure 1. Cyclovoltammograms of  Au solution on n-Si(100): (◻) first scan, (■) subsequent scans, and (▴) gold electrodes. Sweep rate
Au solution on n-Si(100): (◻) first scan, (■) subsequent scans, and (▴) gold electrodes. Sweep rate  .
.
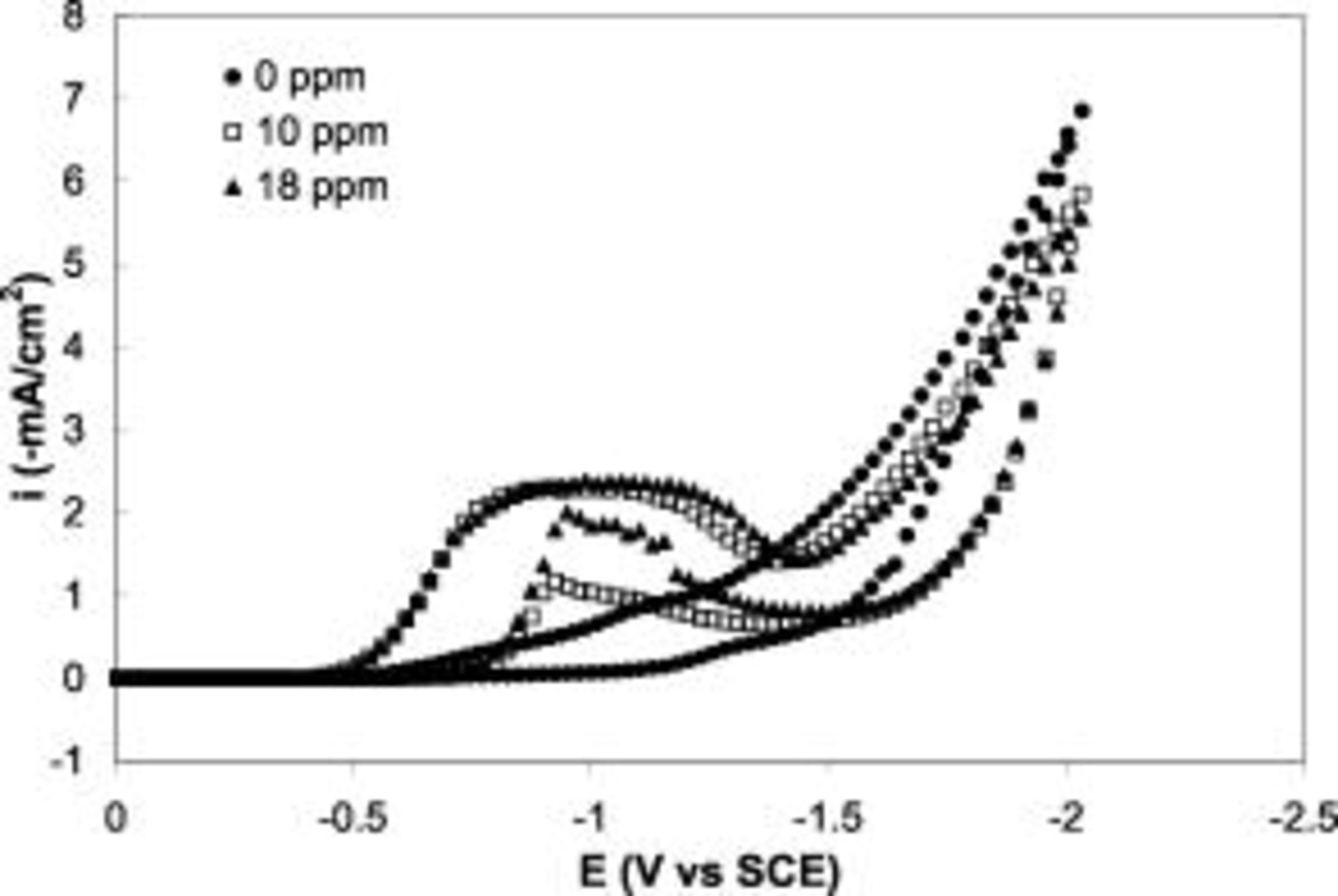
In order to study the effect of thallium on the Au electrodeposition on Si, polarization was carried out in electrolytes with different amounts of thallium(I) acetate at the same conditions as Fig. 1. The first scan is presented in Fig. 2. At a potential more positive than  vs SCE, the addition of Tl increases the current density. As the Tl concentration increases, this current enhancement becomes more pronounced and reaches a "saturation" at
vs SCE, the addition of Tl increases the current density. As the Tl concentration increases, this current enhancement becomes more pronounced and reaches a "saturation" at  Tl, for which a current peak was observed at around
Tl, for which a current peak was observed at around  vs SCE. In a Au-free electrolyte, with only Tl and
vs SCE. In a Au-free electrolyte, with only Tl and  supporting salts, a constant but negligibly small current was observed at low potential up to
supporting salts, a constant but negligibly small current was observed at low potential up to  vs SCE, clearly indicating that there is no significant electrochemical reaction of Tl on Si. Studies in different electrolytes and different agitation conditions showed that the peak current was proportional to the square root of the rotation speed and to the Au concentration, indicative of mass-transport control of Au plating. In this potential range, the presence of Tl greatly catalyzes the Au electrodeposition kinetics, resulting in a mass-transport-limited system.
vs SCE, clearly indicating that there is no significant electrochemical reaction of Tl on Si. Studies in different electrolytes and different agitation conditions showed that the peak current was proportional to the square root of the rotation speed and to the Au concentration, indicative of mass-transport control of Au plating. In this potential range, the presence of Tl greatly catalyzes the Au electrodeposition kinetics, resulting in a mass-transport-limited system.
Figure 2. The first scan of the cyclic voltammograms on n-Si(100) electrode in  Au solutions with (●)
Au solutions with (●)  , (◻)
, (◻)  , and (▴)
, and (▴)  Tl in form thallium(I) acetate. Sweep rate
Tl in form thallium(I) acetate. Sweep rate  .
.
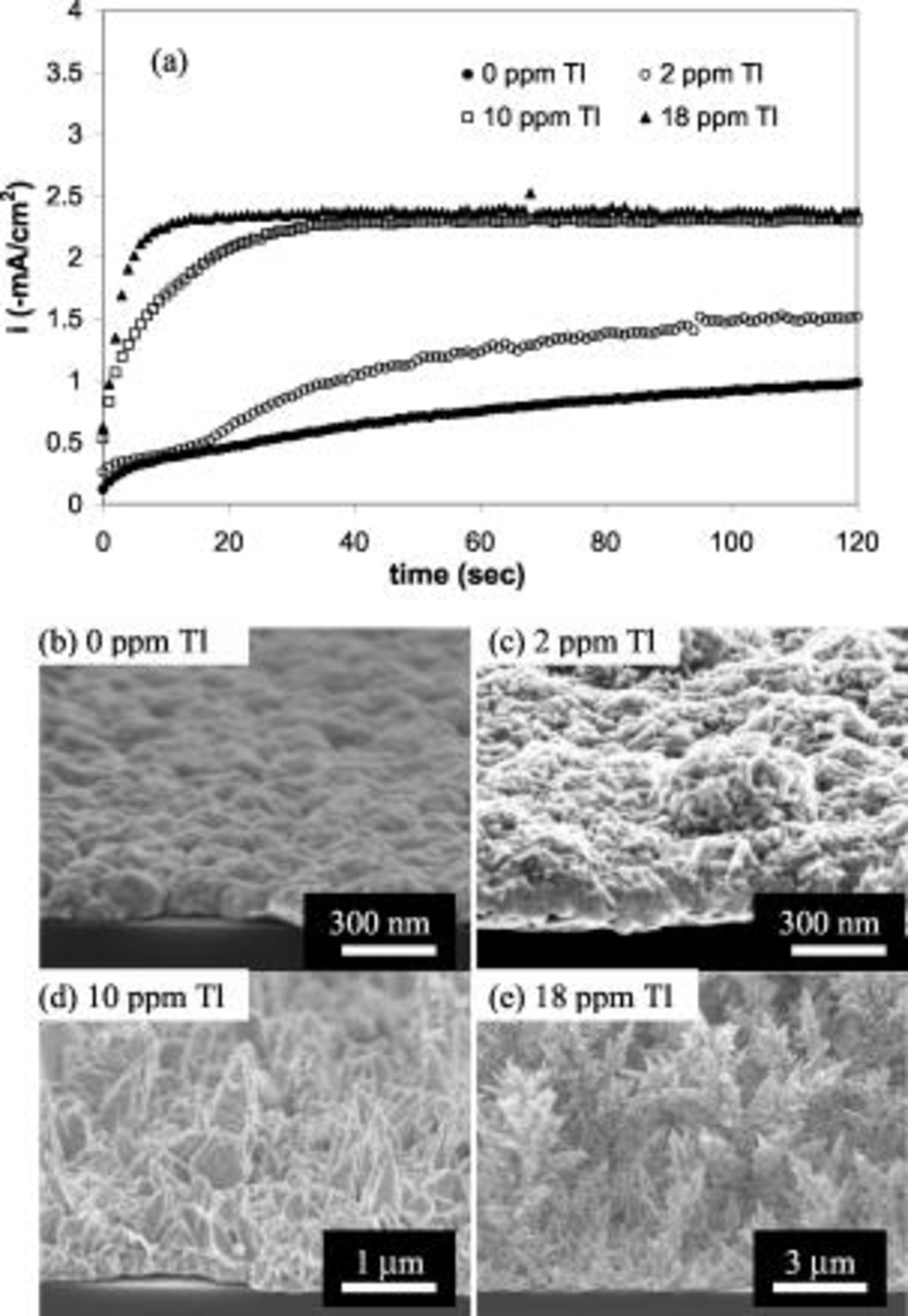
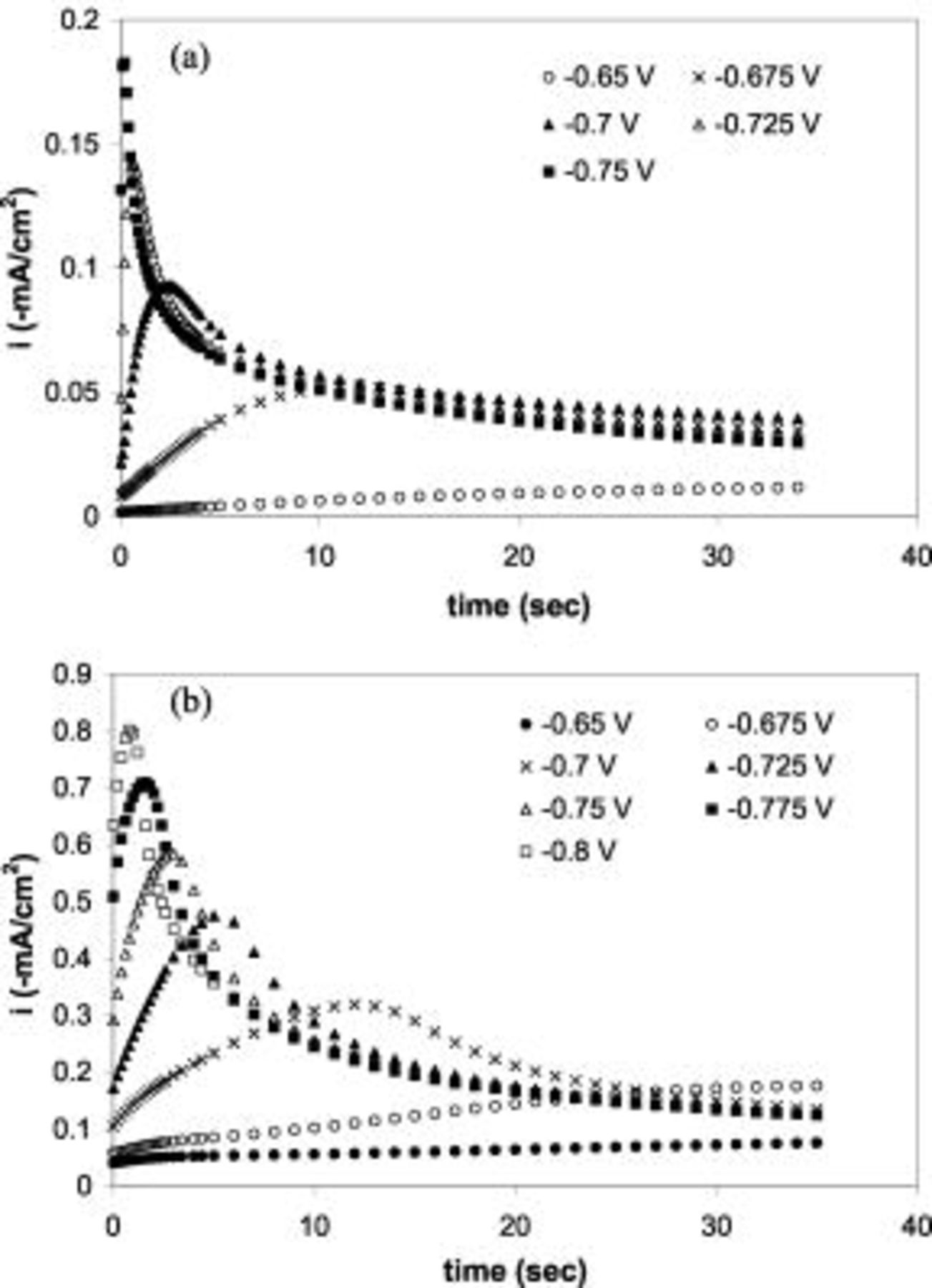
Potentiostatic deposition was carried out at  vs SCE at
vs SCE at  , and the current densities as a function of time are shown in Fig. 3a. A same constant current was again observed for electrolytes with
, and the current densities as a function of time are shown in Fig. 3a. A same constant current was again observed for electrolytes with  Tl or more. The deposition rates obtained from weight difference measurements, 0.04, 0.12, 0.18, and
Tl or more. The deposition rates obtained from weight difference measurements, 0.04, 0.12, 0.18, and  for electrolytes with 0, 2, 10, and
for electrolytes with 0, 2, 10, and  Tl, respectively, are perfectly consistent with the conclusion that Au deposition was facilitated by Tl and that the limiting current was reached at this potential when the Tl concentration reaches
Tl, respectively, are perfectly consistent with the conclusion that Au deposition was facilitated by Tl and that the limiting current was reached at this potential when the Tl concentration reaches  . Film composition was analyzed by photon-induced X-ray emission (PIXE). For the film plated with
. Film composition was analyzed by photon-induced X-ray emission (PIXE). For the film plated with  Tl addition, the Tl inclusion was determined as
Tl addition, the Tl inclusion was determined as  . It is believed that thallium catalyzes the Au deposition, without being codeposited.
. It is believed that thallium catalyzes the Au deposition, without being codeposited.
Figure 3. (a) Current–time curves and (b–e) SEM micrographs of the deposits on n-Si(100) for potentiostatic plating at  vs SCE in
vs SCE in  Au solutions with (●, b)
Au solutions with (●, b)  , (○, c)
, (○, c)  , (◻, d)
, (◻, d)  , and (▴, e)
, and (▴, e) 
 .
.
The films from the potentiostatic deposition were also characterized with scanning electron microscopy (SEM), as shown in Fig. 3b, 3c, 3d and 3e. The addition of Tl in the electrolyte increases the surface roughness. In the extreme case of  Tl, tree-shaped gold deposits were plated. It is well known that mass-transport-controlled electrodeposition generally increases the surface roughness and results in dendritic growth. However, the gold electrodeposition current density in the presence of 18 and
Tl, tree-shaped gold deposits were plated. It is well known that mass-transport-controlled electrodeposition generally increases the surface roughness and results in dendritic growth. However, the gold electrodeposition current density in the presence of 18 and  of Tl were the same while the deposits were much different. Our hypothesis is that a catalytic Tl species adsorbs onto the electrode surface and, based upon its coverage, it catalyzes the Au deposition rate. The local variation in the coverage of the adsorbed Tl species causes a difference in the local deposition rate, resulting in a tree-shaped deposit.
of Tl were the same while the deposits were much different. Our hypothesis is that a catalytic Tl species adsorbs onto the electrode surface and, based upon its coverage, it catalyzes the Au deposition rate. The local variation in the coverage of the adsorbed Tl species causes a difference in the local deposition rate, resulting in a tree-shaped deposit.
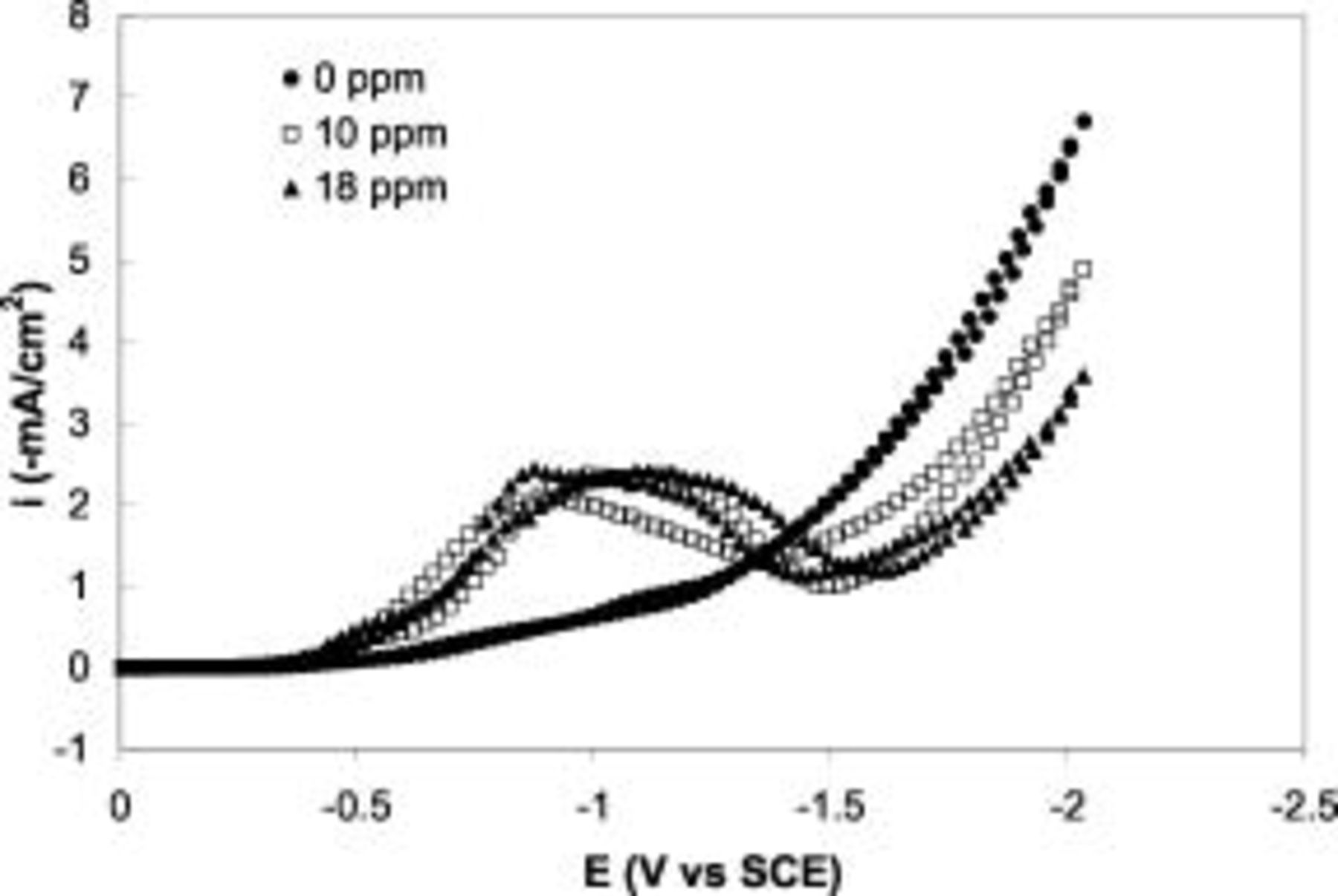
Figure 4 shows the scans after the first scan in cyclic voltammograms of Au electrodeposition on Si with different amounts of Tl. Comparison of the voltammograms in Fig. 2 and 4 shows that the current peak in the first scan is always lower than the ones in subsequent scans. The cathode surface was initially Si and was converted to Au surface by the subsequent deposition. The lower peak current in the first scan suggests that the catalytic effect of Tl on Au deposition on Au is more pronounced than Au deposition on Si. In other words, the Au deposits more preferentially on Au over Si at the low-potential range in the presence of  .
.
Figure 4. Scans after the first scan of the cyclic voltammograms on n-Si(100) electrode in  Au solutions with (●)
Au solutions with (●)  , (◻)
, (◻)  , and (▴)
, and (▴)  Tl in form thallium(I) acetate. Sweep rate
Tl in form thallium(I) acetate. Sweep rate  .
.
In addition to the deposition rate enhancement at low potential, it is also evident in Fig. 4 that the presence of Tl decreases the Au deposition rate at high potential. This current suppression becomes more pronounced as the Tl concentration increases in the electrolyte, and the suppression does not "saturate" even at  Tl. Furthermore, comparison of Fig. 2 and 4 shows that this inhibition effect is more evident on the Au than on the Si surface. Potentiostatic deposition was also carried out at
Tl. Furthermore, comparison of Fig. 2 and 4 shows that this inhibition effect is more evident on the Au than on the Si surface. Potentiostatic deposition was also carried out at  vs SCE at different Tl concentrations for composition analysis with PIXE. The Tl contents were
vs SCE at different Tl concentrations for composition analysis with PIXE. The Tl contents were  and
and  for the deposits with the presence of 2- and
for the deposits with the presence of 2- and  Tl in the electrolyte, respectively. The suppression of Au electrodeposition at high potential is believed to result from the codeposition of Tl. The higher the Tl concentration in the electrolytes, the higher Tl incorporation in the deposit, and the more pronounced the current suppression at high potentials.
Tl in the electrolyte, respectively. The suppression of Au electrodeposition at high potential is believed to result from the codeposition of Tl. The higher the Tl concentration in the electrolytes, the higher Tl incorporation in the deposit, and the more pronounced the current suppression at high potentials.
Electrodeposition of  on Au electrodes was studied decades ago and an underpotential deposition mechanism was suggested.21 The deposition potential shift was up to
on Au electrodes was studied decades ago and an underpotential deposition mechanism was suggested.21 The deposition potential shift was up to  in an acidic Au solution due to the strong interaction between Au and Tl atoms.21, 22 In this study, an adsorbed Tl intermediate apparently catalyzes the deposition of Au on Au surface at low potentials. As the potential increases, the adsorbate is further reduced into Tl atoms. Consequently, the Au electrodeposition becomes no more catalyzed, and the current drops at around
in an acidic Au solution due to the strong interaction between Au and Tl atoms.21, 22 In this study, an adsorbed Tl intermediate apparently catalyzes the deposition of Au on Au surface at low potentials. As the potential increases, the adsorbate is further reduced into Tl atoms. Consequently, the Au electrodeposition becomes no more catalyzed, and the current drops at around  vs SCE in the cyclic voltammograms (Fig. 2 and 4). At the same time, the Tl atom starts to incorporate into the Au deposit, resulting in the suppressed Au deposition.
vs SCE in the cyclic voltammograms (Fig. 2 and 4). At the same time, the Tl atom starts to incorporate into the Au deposit, resulting in the suppressed Au deposition.
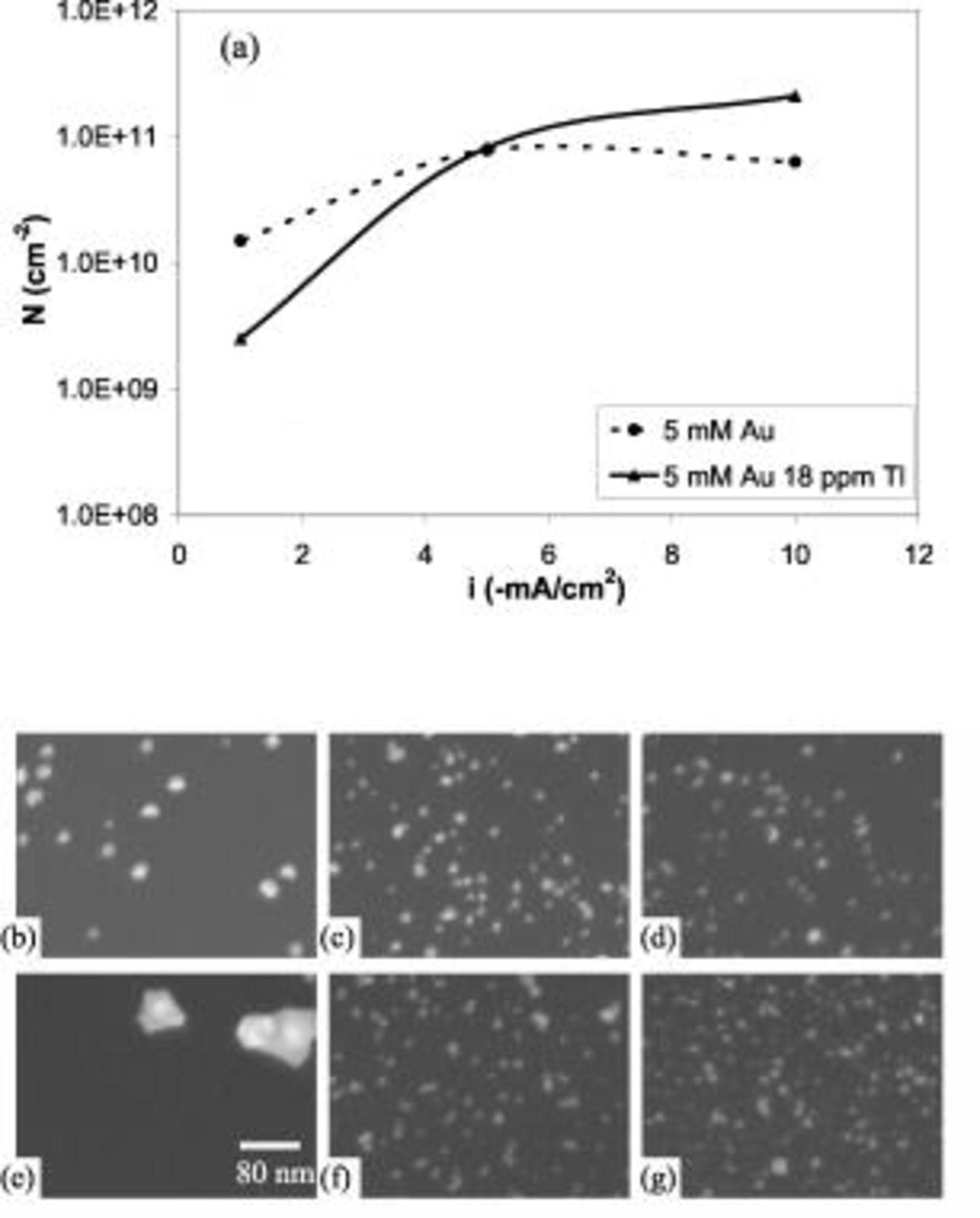
The effect of Tl on the Au nucleation on Si was studied, and Fig. 5 shows the nucleation densities and the SEM micrographs at different galvanostatic plating in the absence and presence of 
 . A rotation rate of
. A rotation rate of  was used. In either thallium-free or thallium-containing electrolytes, higher current density results in smaller nuclei and higher nucleation density, as expected from the nucleation overpotential observed for Au deposition on Si (Fig. 1). Comparison of the two electrolytes shows that the presence of
was used. In either thallium-free or thallium-containing electrolytes, higher current density results in smaller nuclei and higher nucleation density, as expected from the nucleation overpotential observed for Au deposition on Si (Fig. 1). Comparison of the two electrolytes shows that the presence of 
 significantly changed the Au nucleation on Si. When a low current density is applied, such as
significantly changed the Au nucleation on Si. When a low current density is applied, such as  , the nuclei size is increased and the nucleation density is decreased by
, the nuclei size is increased and the nucleation density is decreased by  , shown in Fig. 5b and 5e. The previous discussion showed that the Au plating at low potential was catalyzed by
, shown in Fig. 5b and 5e. The previous discussion showed that the Au plating at low potential was catalyzed by  on Au but not on Si (Fig. 2 and 4). Therefore, once a Au particle forms, the subsequent Au deposition preferably occurs on the Au particle in galvanostatic plating, resulting in a larger particle size and a lower nucleation density.
on Au but not on Si (Fig. 2 and 4). Therefore, once a Au particle forms, the subsequent Au deposition preferably occurs on the Au particle in galvanostatic plating, resulting in a larger particle size and a lower nucleation density.
Figure 5. Nucleation density of galvanostatic plating in n-Si(100) in  Au solutions: (a) summary, (b–d)
Au solutions: (a) summary, (b–d)  Tl at
Tl at  ,
,  , and
, and  , (e–g)
, (e–g)  Tl at
Tl at  ,
,  , and
, and  .
.
On the contrary, Au deposition on Au is suppressed at a high potential due to the codeposition of Tl, and this suppression was less pronounced for Au deposition on Si. Consequently, galvanostatic plating at a high current density, such as  , in a Tl-containing electrolyte is expected to result in a higher nucleation density than Tl-free electrolyte, as proved in Fig. 5d and 5g. At a medium current, such as
, in a Tl-containing electrolyte is expected to result in a higher nucleation density than Tl-free electrolyte, as proved in Fig. 5d and 5g. At a medium current, such as  , shown in Fig. 5c and 5f, no significant difference was observed for the nucleation in the absence and presence of
, shown in Fig. 5c and 5f, no significant difference was observed for the nucleation in the absence and presence of  . The effects of
. The effects of  on Au nucleation density are summarized in Fig. 5a. The highest nucleation density of
on Au nucleation density are summarized in Fig. 5a. The highest nucleation density of  was achieved at high current densities when
was achieved at high current densities when 
 was present.
was present.
Due to the weak interaction between metal and Si substrate, metal deposition on Si follows the Volmer–Weber model, an island formation and a mass-transport-controlled 3D growth process. For the two extreme cases, ideal progressive and instantaneous nucleation, the current transient response for a potential step on a stagnant electrode is described by the following equations2 instantaneous nucleation
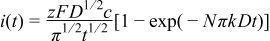
progressive nucleation
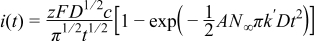
where  and
and  . A simplified form can be obtained by nondimensionalizing the current and time with the values at maximum current,
. A simplified form can be obtained by nondimensionalizing the current and time with the values at maximum current,  and
and  2, 3instantaneous nucleation
2, 3instantaneous nucleation
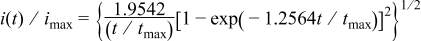
progressive nucleation
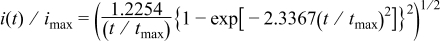
Figure 6a and 6b shows the stagnant chronoamperometry in  Au electrolytes without and with
Au electrolytes without and with 
 , respectively. At a potential more negative than
, respectively. At a potential more negative than  vs SCE a current peak was observed in the thallium-free electrolyte, after which the current decays to the same value for all potentials. In the presence of
vs SCE a current peak was observed in the thallium-free electrolyte, after which the current decays to the same value for all potentials. In the presence of  Tl, not only a significantly higher peak current but also a delay of the peak current was observed, suggesting a second current transient, which was believed to be the adsorption of
Tl, not only a significantly higher peak current but also a delay of the peak current was observed, suggesting a second current transient, which was believed to be the adsorption of  during the Au electrodeposition.
during the Au electrodeposition.
Figure 6. Current transients in stagnant potentiostatic plating on n-Si(100) at different potentials in  Au solutions with (a)
Au solutions with (a)  and (b)
and (b)  Tl.
Tl.
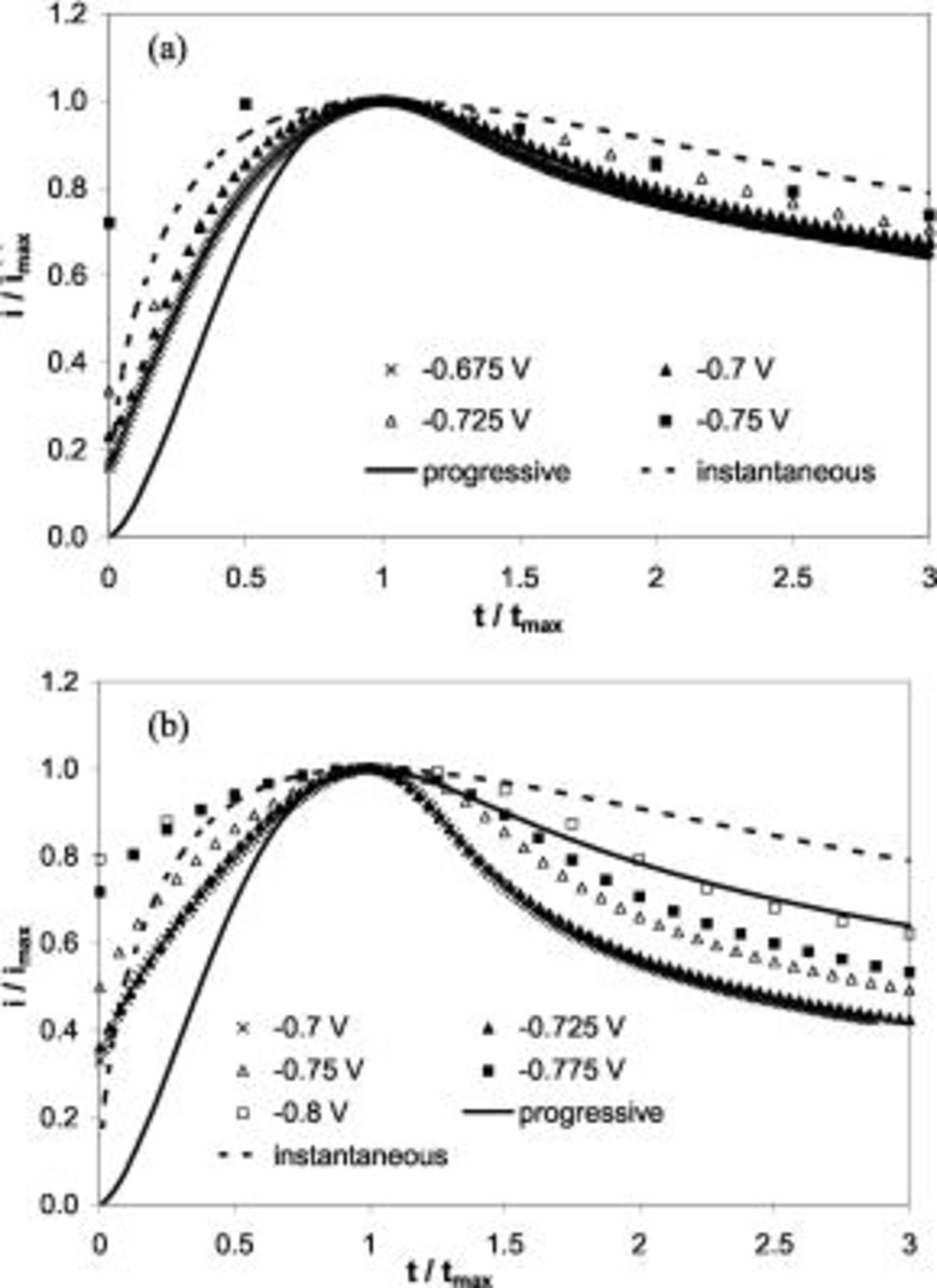
The dimensionless current transients were replotted in Fig. 7a and 7b for the potentials where a current maxima were observed. The ideal progressive and instantaneous nucleation cases were included for comparison. It can be seen that the nucleation in a thallium-free  Au electrolyte at low potential,
Au electrolyte at low potential,  vs SCE, is between the progressive and instantaneous cases. When the potential reaches
vs SCE, is between the progressive and instantaneous cases. When the potential reaches  vs SCE, the current transient falls out of the range of the two extreme cases, indicative of other side reactions. The nonuniform nuclei size in the SEM micrographs in Fig. 5 also confirmed the progressive nucleation process.
vs SCE, the current transient falls out of the range of the two extreme cases, indicative of other side reactions. The nonuniform nuclei size in the SEM micrographs in Fig. 5 also confirmed the progressive nucleation process.
Figure 7. Normalized current transients in stagnant potentiostatic plating on n-Si(100) at different potentials in  Au solutions with (a)
Au solutions with (a)  and (b)
and (b)  Tl.
Tl.
On the contrary, the presence of 
 results in a current transient outside the two extreme cases for all the potentials studied. In general, the current is higher than the instantaneous nucleation before the current maxima but lower than the progressive case after the maxima, consistent with an adsorption-saturation hypothesis of the Tl adsorbate on Au. For a Langmuir adsorption process, where the surface active sites can only be occupied by one monolayer of adsorbate, the adsorption rate is proportional to the free sites and the current from the adsorption of charged species decays to zero as all sites are occupied. This capacitor-like effect is believed to introduce a current transient, which increases the current before the maximum and decays to zero after the maximum.
results in a current transient outside the two extreme cases for all the potentials studied. In general, the current is higher than the instantaneous nucleation before the current maxima but lower than the progressive case after the maxima, consistent with an adsorption-saturation hypothesis of the Tl adsorbate on Au. For a Langmuir adsorption process, where the surface active sites can only be occupied by one monolayer of adsorbate, the adsorption rate is proportional to the free sites and the current from the adsorption of charged species decays to zero as all sites are occupied. This capacitor-like effect is believed to introduce a current transient, which increases the current before the maximum and decays to zero after the maximum.
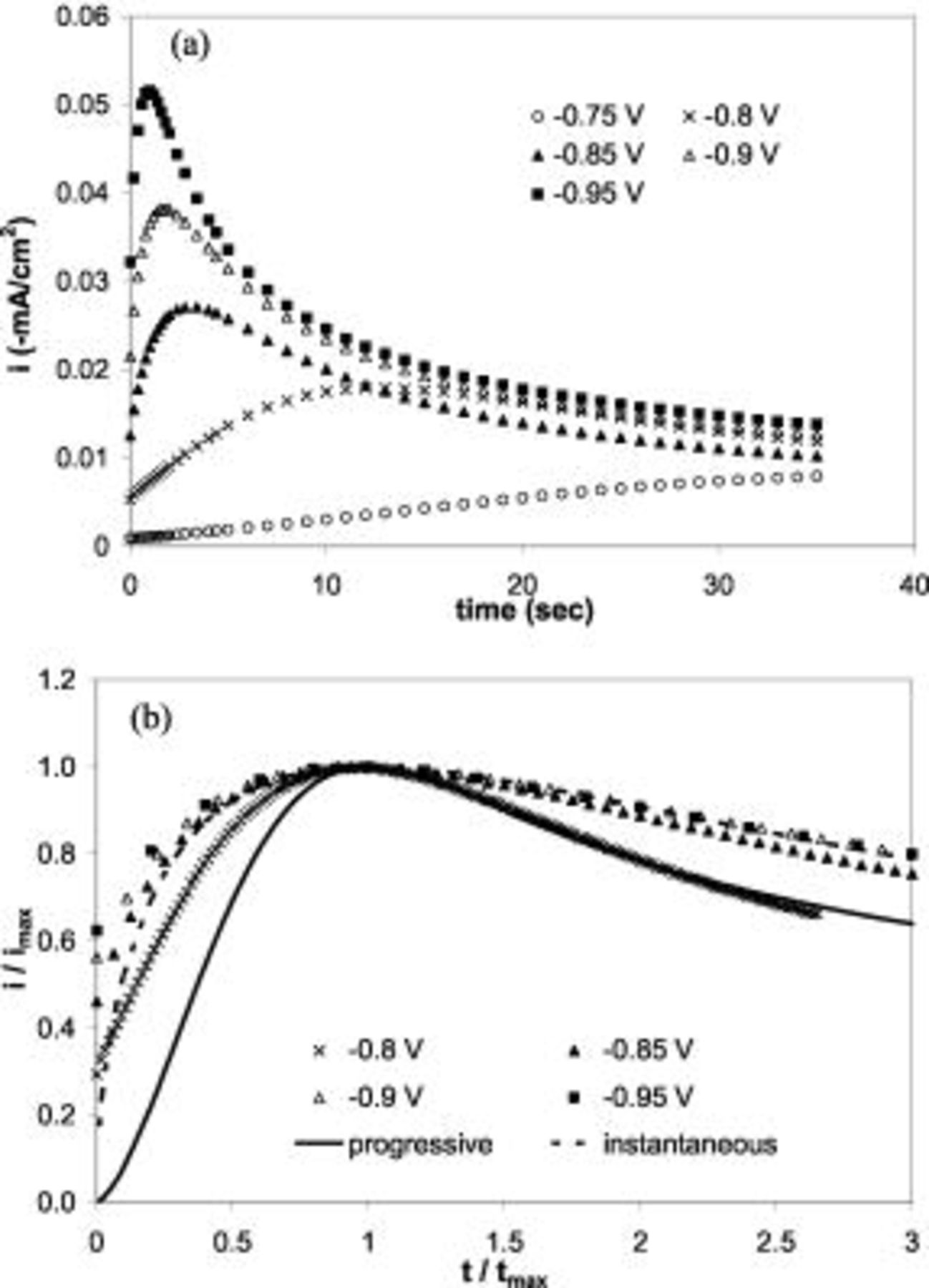
To achieve a high nucleation density with uniform nuclei size, instantaneous nucleation process is preferred. Electrolytes with different gold concentrations were studied for the nucleation process. Figure 8a and 8b presents the chronoamperometry in a Tl-free electrolyte with  Au. For potentials higher than
Au. For potentials higher than  vs SCE, the nucleation was found to be close to an instantaneous process. Figure 9a and 9b shows SEM micrographs for Au deposition at
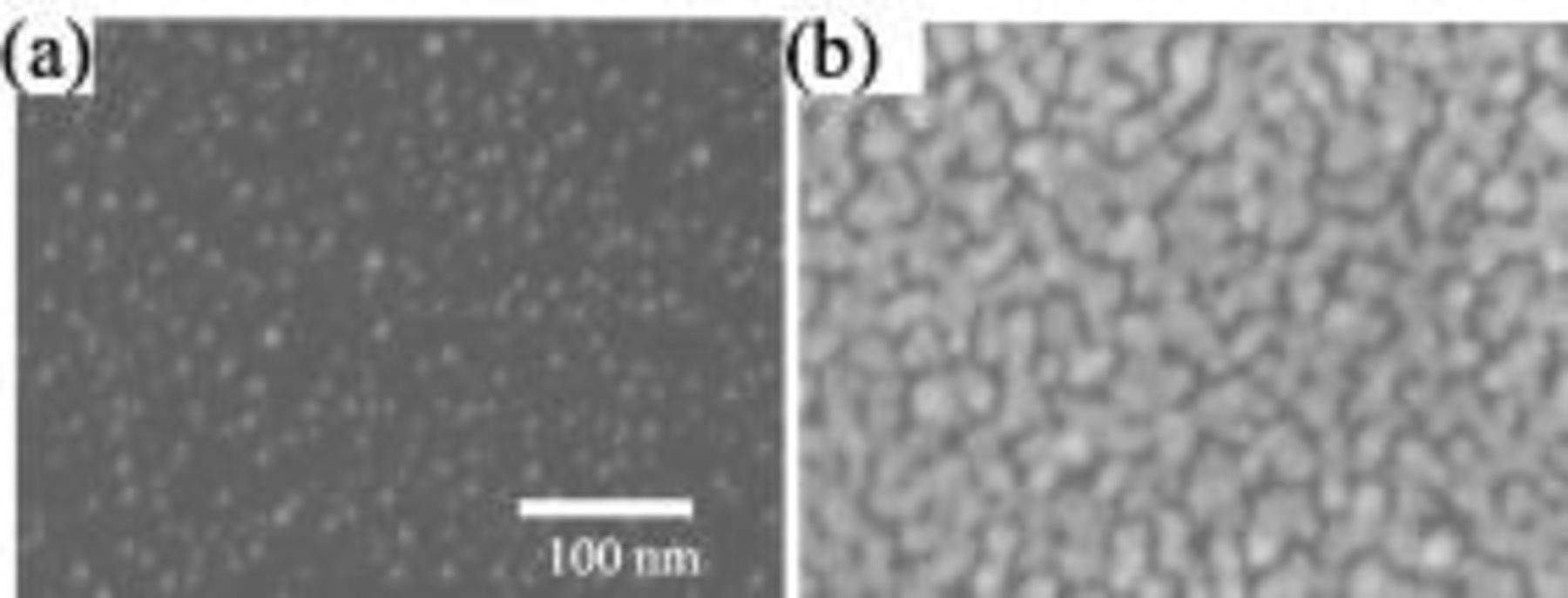
vs SCE, the nucleation was found to be close to an instantaneous process. Figure 9a and 9b shows SEM micrographs for Au deposition at  in this dilute Au electrolyte. The nucleation density in Fig. 9a,
in this dilute Au electrolyte. The nucleation density in Fig. 9a,  , was found much higher than the case of
, was found much higher than the case of  Au electrolyte,
Au electrolyte,  . As the deposition continues for a longer time, a uniform nuclei size was maintained and finally merged with each other. No new nuclei were formed for the
. As the deposition continues for a longer time, a uniform nuclei size was maintained and finally merged with each other. No new nuclei were formed for the  Au case, consistent with the instantaneous nucleation process observed in the chronoamperometry study.
Au case, consistent with the instantaneous nucleation process observed in the chronoamperometry study.
Figure 8. (a) Non-normalized and (b) normalized current transients in stagnant potentiostatic plating on n-Si(100) at different potentials in  Au solution without Tl.
Au solution without Tl.
Figure 9. SEM micrograph of Au nucleation on n-Si(100) in  Au solution in galvanostatic plating at
Au solution in galvanostatic plating at  for (a) 50 and (b)
for (a) 50 and (b)  .
.
Conclusions
Electrodeposition of Au on n-Si(100) surface was investigated in a mild alkaline chemistry. Addition of  in the electrolyte significantly enhanced the Au deposition rate at low potentials, while the deposition at high potentials was suppressed. An adsorbed Tl intermediate is proposed to explain both the catalytic and the suppressive effects on Au electrodeposition. The nucleation of Au on Si was found to be dependent on the Au concentration and applied potential. An instantaneous nucleation process was observed for a
in the electrolyte significantly enhanced the Au deposition rate at low potentials, while the deposition at high potentials was suppressed. An adsorbed Tl intermediate is proposed to explain both the catalytic and the suppressive effects on Au electrodeposition. The nucleation of Au on Si was found to be dependent on the Au concentration and applied potential. An instantaneous nucleation process was observed for a  Au electrolyte, while the nucleation was found between instantaneous and progressive processes for
Au electrolyte, while the nucleation was found between instantaneous and progressive processes for  Au electrolyte. The addition of Tl decreases the nucleation density and increases the nuclei size at low current, while it increases the nucleation density and decreases the size at high current.
Au electrolyte. The addition of Tl decreases the nucleation density and increases the nuclei size at low current, while it increases the nucleation density and decreases the size at high current.
Acknowledgments
The authors would like to thank Dr. Guy Cohen for helpful discussions and Dr. Andrew Kellock for composition analysis.
IBM, T. J. Watson Research Center assisted in meeting the publication costs of this article.