Abstract
Silicon and titanium boride nanocomposites were synthesized using high-energy mechanical milling. The nanocomposites obtained after mechanical milling consist of amorphous silicon and nanocrystalline titanium boride. The nanocomposite containing 40 mol % silicon obtained after milling for 20 h exhibits a stable capacity of  X-ray diffraction and scanning electron microscopy analyses indicated that the nanocomposite retains its initial phase and microstructure during electrochemical cycling. Premilling of the inactive
X-ray diffraction and scanning electron microscopy analyses indicated that the nanocomposite retains its initial phase and microstructure during electrochemical cycling. Premilling of the inactive  component appears to increase the stability of the capacity of the nanocomposite electrodes due to a probable homogeneous distribution of the induced stress during cycling. © 2003 The Electrochemical Society. All rights reserved.
component appears to increase the stability of the capacity of the nanocomposite electrodes due to a probable homogeneous distribution of the induced stress during cycling. © 2003 The Electrochemical Society. All rights reserved.
Export citation and abstract BibTeX RIS
Li-ion batteries continue to be the state-of-the-art power sources for consumer electronic devices because of their high volumetric and gravimetric capacities. However, while cathode systems have been researched extensively, anode materials are now beginning to receive significant attention.1
2
3
4
5
6
7
8 While graphite has been typically the anode material of choice, metals and metallic alloys have been studied as anodes9
10
11 because of their higher theoretical capacity than graphitic materials. Among the metals, which form Li-alloys, Sn and Si are well known due to their high capacity 4000 and 990 mAh/g, respectively.12 Despite the high capacity, metallic systems pose a major problem preventing their use as anodes in commercial systems. This is due mainly to the very large change in volume during charge/discharge cycles resulting in cracking or crumbling of the anodes causing loss in capacity during cycling. Studies have been conducted to overcome this problem. Most current studies on anode materials other than carbon have focused on creating a composite microstructure comprising an inactive host matrix containing a finely dispersed active phase. A nanocrystalline state helps to preserve the crystallographic stability minimizing the drastic effects of the large volumetric changes probably due to the reduced density of atoms in a nanosized grain.13 Mao and Dahn have also reported the potential of Sn-Fe-C based nanocomposite systems, while Thackeray and co-workers have studied the  and InSb systems for anode applications.12
14
15
16
17 Similarly, SnSb-based systems have also been investigated.18 All these nanocomposite systems are based on a concept analogous to tin oxide glasses wherein the electrochemically active phase is generated after electrochemical insertion of lithium. Although these systems are promising, there are problems related to either capacity or cyclability. The chemical and electrochemical stability are also difficult to control in these systems. As a result, there is a need for improving the demonstrated concept of "active-inactive" nanocomposites further. One possibility is the direct synthesis of nanocomposites based on active and inactive phases. Such a nanocomposite offers better flexibility with regard to control of the initial composition which remains invariant.
and InSb systems for anode applications.12
14
15
16
17 Similarly, SnSb-based systems have also been investigated.18 All these nanocomposite systems are based on a concept analogous to tin oxide glasses wherein the electrochemically active phase is generated after electrochemical insertion of lithium. Although these systems are promising, there are problems related to either capacity or cyclability. The chemical and electrochemical stability are also difficult to control in these systems. As a result, there is a need for improving the demonstrated concept of "active-inactive" nanocomposites further. One possibility is the direct synthesis of nanocomposites based on active and inactive phases. Such a nanocomposite offers better flexibility with regard to control of the initial composition which remains invariant.
To preserve and stabilize the original morphological state of the anode and thereby attain good electrochemical properties, various material systems other than oxide-based composites have been analyzed. The main goal is to minimize the mechanical stress induced by the large ensuing volumetric changes undergone by the active phase. Based on the results of our previous study involving Si and nanocrystalline TiN,19 it is clear that TiN exhibits good properties to justify its use as an inactive matrix in nanocomposite anodes. Thus materials with good electrical conductivity, mechanical strength, and chemical inertness are needed to provide good binding properties and also withstand the stresses attributed to alloying and dealloying of the active element with Li. Titanium boride is also known to exhibit properties similar to titanium nitride, and could also act as a potential inactive matrix for silicon. Therefore, in this paper we explore the concept of active-inactive nanocomposites already demonstrated in Si and TiN further by synthesizing nanocomposites of silicon and titanium boride using high-energy mechanical milling (HEMM). Processing variables such as milling time and the effect of initial particle size of the inactive component on the electrochemical capacity and stability of the anode have been investigated. Experimental studies and results of the structural and electrochemical analyses are presented indicating its promising nature. In addition, microstructural and phase analyses have been conducted to identify the optimum experimental conditions necessary for attaining high capacity.
Experimental
Nanocomposites of Si and  were prepared using a SPEX-8000 high-energy mechanical mill. Commercial elemental powder of Si (Aldrich, 99.9%) and
were prepared using a SPEX-8000 high-energy mechanical mill. Commercial elemental powder of Si (Aldrich, 99.9%) and  (Aldrich, 99%)) were used as starting materials. Stoichiometric amounts of the powders were weighed and loaded into a hardened steel vial containing hardened steel balls. All the processes prior to milling were conducted inside the glove box (VAC Atmospheres, Hawthorn, CA) and the vial was firmly sealed to prevent and minimize any oxidation of the starting materials.
(Aldrich, 99%)) were used as starting materials. Stoichiometric amounts of the powders were weighed and loaded into a hardened steel vial containing hardened steel balls. All the processes prior to milling were conducted inside the glove box (VAC Atmospheres, Hawthorn, CA) and the vial was firmly sealed to prevent and minimize any oxidation of the starting materials.
To evaluate the electrochemical characteristics, electrodes were fabricated using the as-milled powder by mixing the composition of 87.1 wt % active powder and 7.3 wt % acetylene black. A solution containing 5.6 wt % polyvinylidene fluoride (PVDF) in 1-methyl-2-pyrrolidinone (NMP) was added to the mixture. The as-prepared solution was coated onto a Cu foil. A hockey puck cell design was used employing lithium foil as an anode and 1 M  in ethylene carbonate (EC)/dimethyl carbonate (DMC) (2:1) as the electrolyte. All the batteries tested in this study were cycled from 0.02 to 1.2 V employing a current density of
in ethylene carbonate (EC)/dimethyl carbonate (DMC) (2:1) as the electrolyte. All the batteries tested in this study were cycled from 0.02 to 1.2 V employing a current density of  which corresponds to a C rate in the range of C/20 to C/25, and a minute rest period between the charge/discharge cycles using a potentiostat (Arbin electrochemical instrument). The phase(s) present in the as-milled powders and the cycled electrode were analyzed using X-ray diffraction (XRD, Rigaku, θ/θ diffractometer) while the nanoscale microstructure was examined using a scanning electron microscope [SEM, Philips XL30, equipped with energy dispersive X-ray (EDX)] and high-resolution transmission electron microscope (HRTEM, JEOL 4000EX). The Philips X'PERT PRO XRD system, equipped with the state-of-the-art detector, X'celerator, which allows the collection of X-ray data 100-fold faster than normal typical detectors was also used to obtain high-resolution XRD spectra at a scan rate of 0.013°/s.
which corresponds to a C rate in the range of C/20 to C/25, and a minute rest period between the charge/discharge cycles using a potentiostat (Arbin electrochemical instrument). The phase(s) present in the as-milled powders and the cycled electrode were analyzed using X-ray diffraction (XRD, Rigaku, θ/θ diffractometer) while the nanoscale microstructure was examined using a scanning electron microscope [SEM, Philips XL30, equipped with energy dispersive X-ray (EDX)] and high-resolution transmission electron microscope (HRTEM, JEOL 4000EX). The Philips X'PERT PRO XRD system, equipped with the state-of-the-art detector, X'celerator, which allows the collection of X-ray data 100-fold faster than normal typical detectors was also used to obtain high-resolution XRD spectra at a scan rate of 0.013°/s.
Results and Discussion
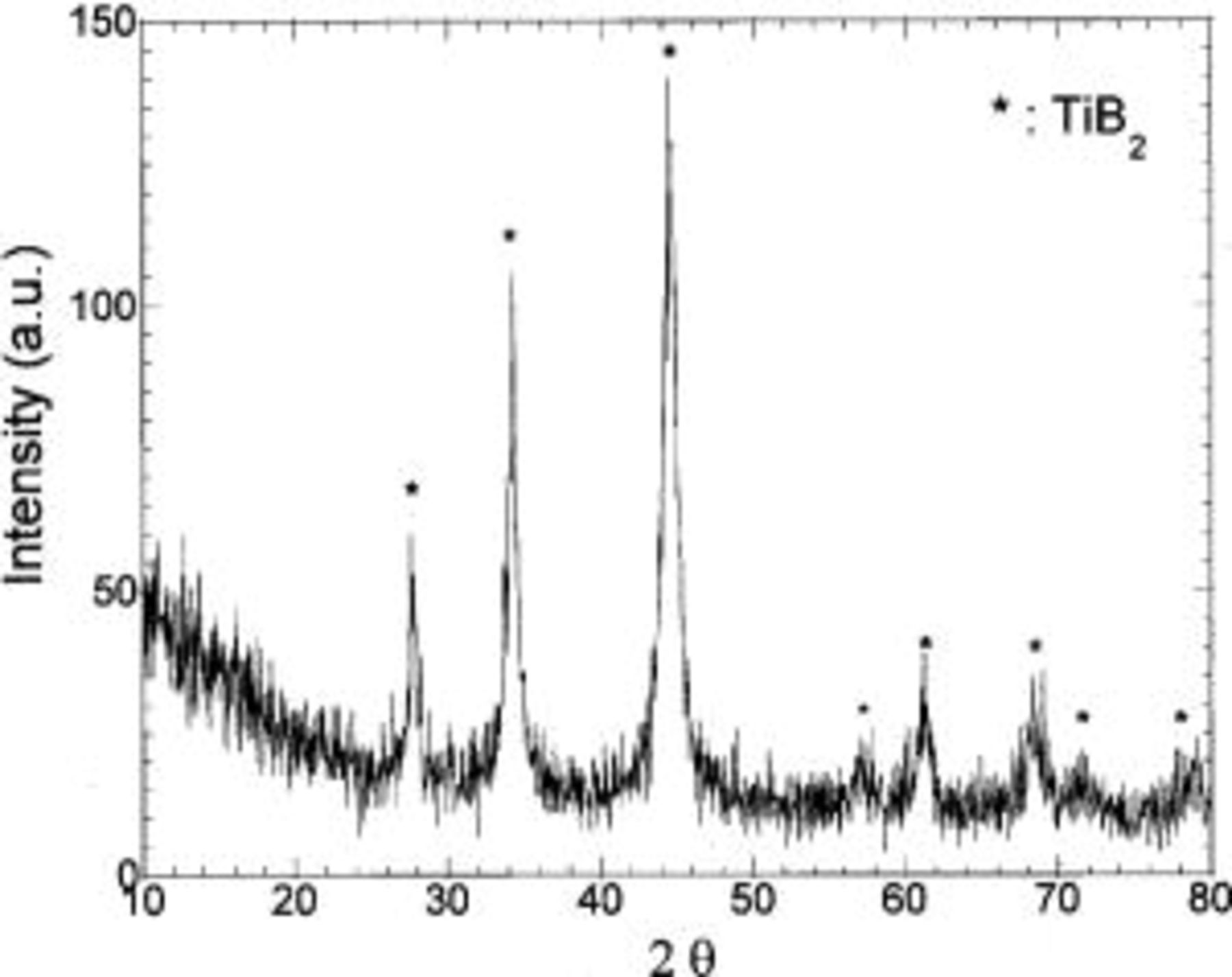
In the Si/TiN nanocomposites, we have shown that HEMM renders the silicon amorphous.19 XRD analysis conducted on the  system also indicates that Si is amorphous even after milling for 6 h (Fig. 1). All the peaks in the patterns correspond to
system also indicates that Si is amorphous even after milling for 6 h (Fig. 1). All the peaks in the patterns correspond to  and the broad nature of the peaks are clearly indicative of the nanocrystalline nature of the powder. The reason for the formation of nanocrystalline or amorphous Si after milling can be attributed to the mechanical strength and hardness of TiN and
and the broad nature of the peaks are clearly indicative of the nanocrystalline nature of the powder. The reason for the formation of nanocrystalline or amorphous Si after milling can be attributed to the mechanical strength and hardness of TiN and  Because
Because  particles act as the milling media, the strength and hardness of the inactive phase accelerates the pulverization of silicon to form very fine amorphous particles.
particles act as the milling media, the strength and hardness of the inactive phase accelerates the pulverization of silicon to form very fine amorphous particles.
Figure 1. XRD patterns of  composites obtained after milling for 6 h.
composites obtained after milling for 6 h.
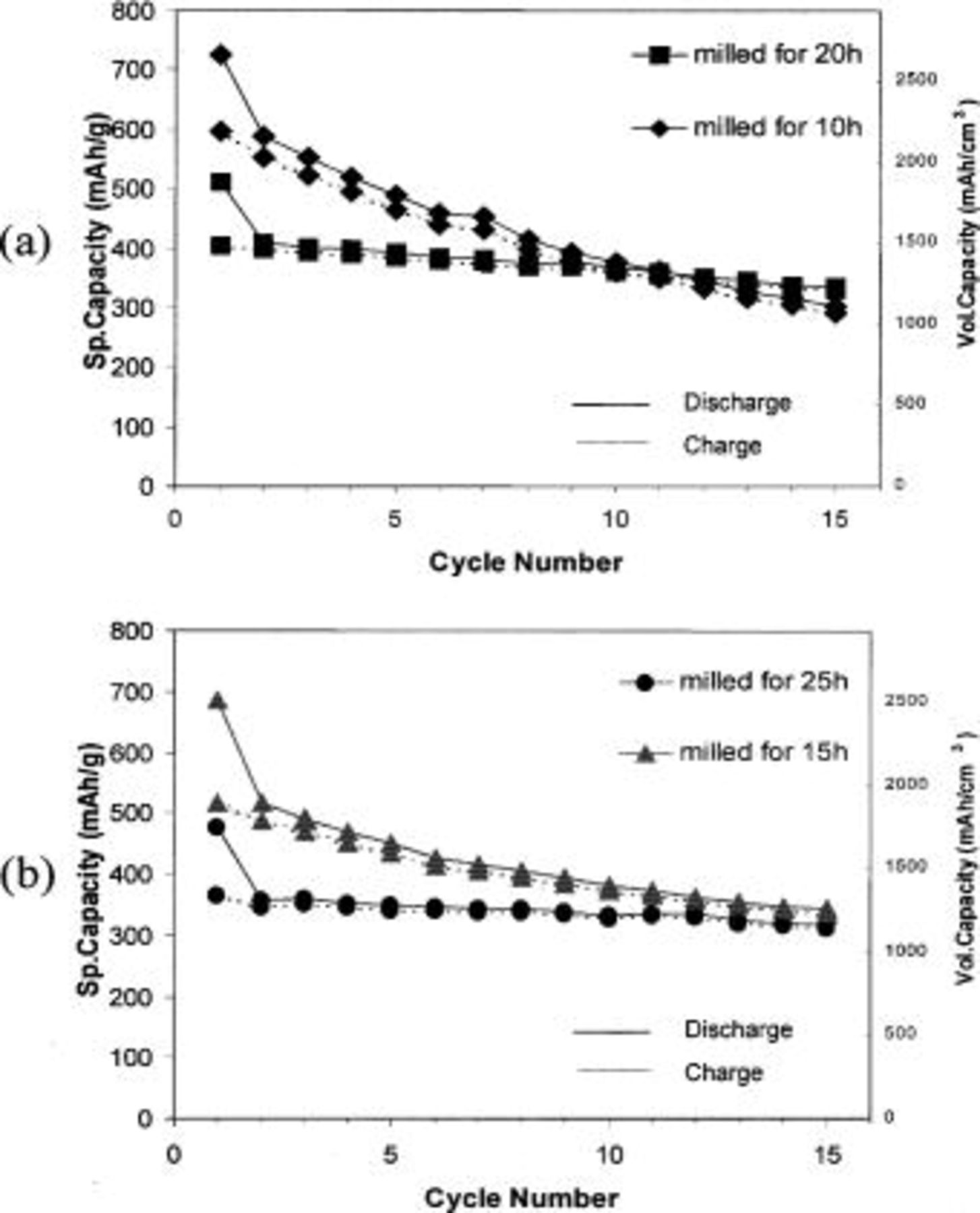
The specific gravimetric and the equivalent volumetric capacity of the electrode prepared with the  nanocomposites containing 40 mol % Si are shown in Fig. 2a and b. In the electrode comprising the nanocomposite obtained after milling for 10 h, the highest first discharge capacity (726 mAh/g) is observed but the capacity fades rapidly. Furthermore, this value is still lower than the theoretical capacity (891 mAh/g) of the
nanocomposites containing 40 mol % Si are shown in Fig. 2a and b. In the electrode comprising the nanocomposite obtained after milling for 10 h, the highest first discharge capacity (726 mAh/g) is observed but the capacity fades rapidly. Furthermore, this value is still lower than the theoretical capacity (891 mAh/g) of the  nanocomposites comprising 40 mol % Si. The nanocomposite obtained after milling for 20 h nevertheless shows an initial capacity as high as 404 mAh/g at the second discharge, which decreases to
nanocomposites comprising 40 mol % Si. The nanocomposite obtained after milling for 20 h nevertheless shows an initial capacity as high as 404 mAh/g at the second discharge, which decreases to  at the end of 15 cycles corresponding to a 1.1% loss/cycle, which is slightly higher than that of Si/TiN
at the end of 15 cycles corresponding to a 1.1% loss/cycle, which is slightly higher than that of Si/TiN  loss /cycle) as reported in Ref. 19. However, the electrodes were cycled using a C rate of C/10 in the Si and TiN. It is therefore likely that the electrochemical response of
loss /cycle) as reported in Ref. 19. However, the electrodes were cycled using a C rate of C/10 in the Si and TiN. It is therefore likely that the electrochemical response of  can also possibly be affected by the different current rate of
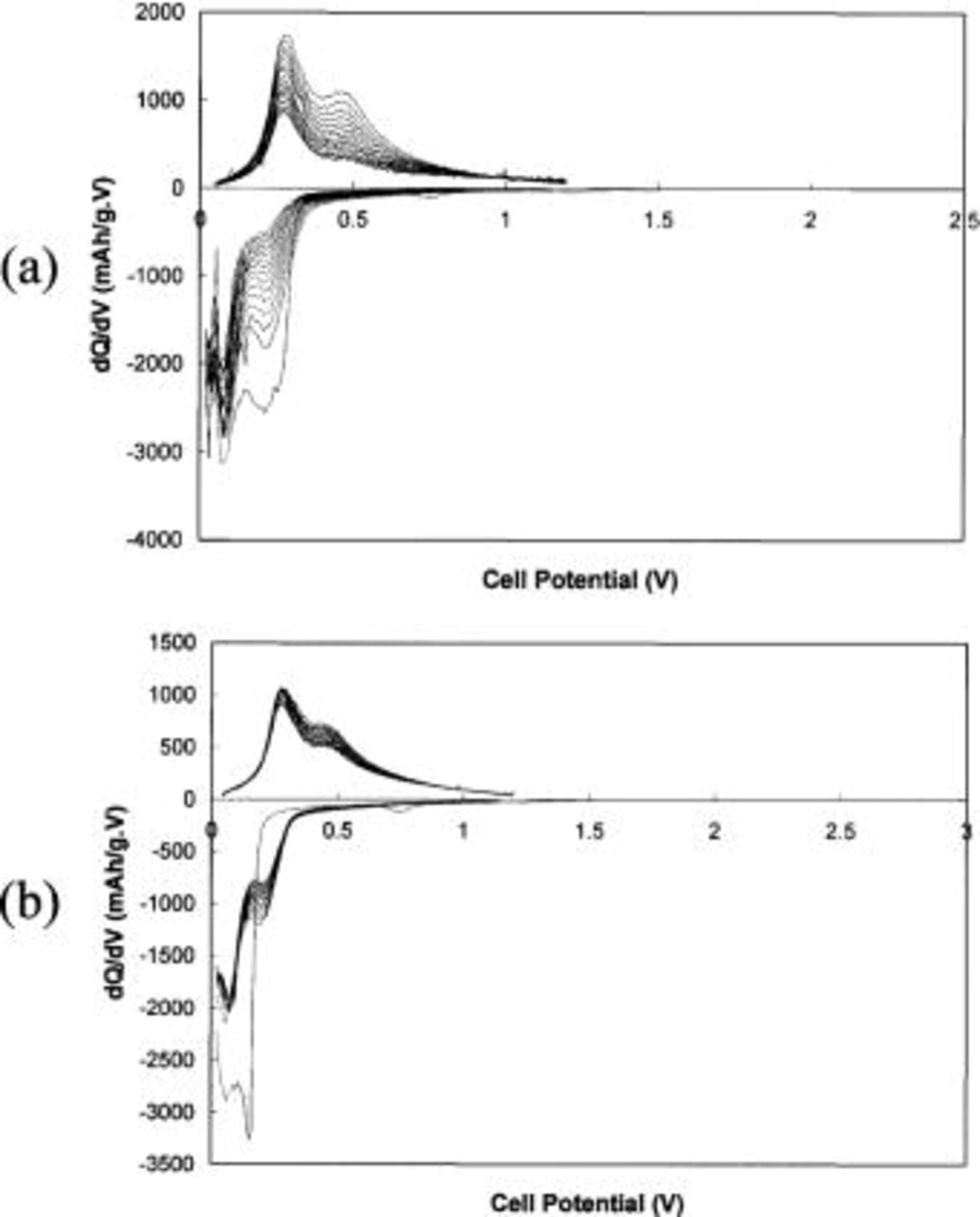
can also possibly be affected by the different current rate of  that was employed here. It should also be noted that the overall capacity appears to decrease as the milling time is increased, indicating a reduction in the amount of the active phase of silicon. Although the reason for the capacity reduction with increasing milling time is not clear yet, one possible explanation is the embedding of Si or the diffusion of Si into the inactive matrix particularly at the active-inactive matrix interface forming a secondary phase. Figure 3a and b shows the differential capacity vs. voltage for 15 cycles for the nanocomposites containing 40 mol % of Si obtained after milling for 10 and 20 h, respectively. Due to the amorphous/nanocrystalline nature of Si, the reaction of Li with Si is represented as broad peaks in each plot. The electrode containing the nanocomposite obtained after milling for 10 h exhibits a reduction in peak intensity with progressive cycles, indicating fade in capacity.
that was employed here. It should also be noted that the overall capacity appears to decrease as the milling time is increased, indicating a reduction in the amount of the active phase of silicon. Although the reason for the capacity reduction with increasing milling time is not clear yet, one possible explanation is the embedding of Si or the diffusion of Si into the inactive matrix particularly at the active-inactive matrix interface forming a secondary phase. Figure 3a and b shows the differential capacity vs. voltage for 15 cycles for the nanocomposites containing 40 mol % of Si obtained after milling for 10 and 20 h, respectively. Due to the amorphous/nanocrystalline nature of Si, the reaction of Li with Si is represented as broad peaks in each plot. The electrode containing the nanocomposite obtained after milling for 10 h exhibits a reduction in peak intensity with progressive cycles, indicating fade in capacity.
Figure 2. Capacity as a function of cycle number for  nanocomposites containing 40 mol % Si obtained after milling for (a) 10, 20 h and (b) 15, 25 h each. Current rate:
nanocomposites containing 40 mol % Si obtained after milling for (a) 10, 20 h and (b) 15, 25 h each. Current rate:  potential: 0.02-1.2 V.
potential: 0.02-1.2 V.
Figure 3. Differential capacity vs. cell potential curves for the first fifteen cycles of the nanocomposite containing  molar ratio obtained after milling for (a) 10 and (b) 20 h, respectively. Current rate:
molar ratio obtained after milling for (a) 10 and (b) 20 h, respectively. Current rate:  potential: 0.02-1.2 V.
potential: 0.02-1.2 V.
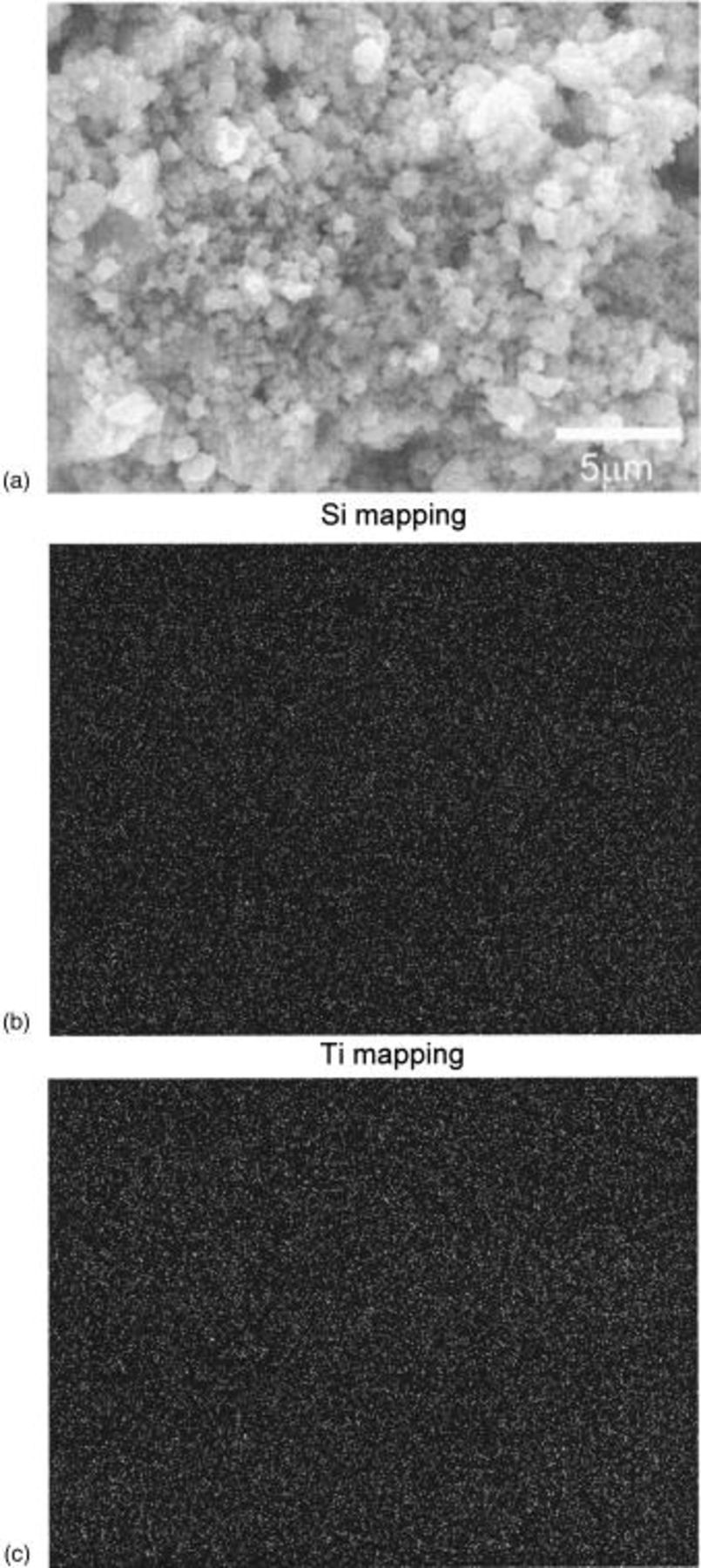
SEM analysis was conducted on the  nanocomposite containing 40 mol % Si obtained after milling for 20 h to analyze the microstructure (Fig. 4a). The size of the agglomerates are in the range of 0.3-3 μm. The morphology of the powder is characteristic of HEMM derived materials comprising brittle precursors.13 EDX mapping of the different elements was conducted to analyze the distribution of the species within the agglomerated particle. EDX analyses were subsequently conducted to analyze silicon and titanium in the powders containing 40 mol % Si
nanocomposite containing 40 mol % Si obtained after milling for 20 h to analyze the microstructure (Fig. 4a). The size of the agglomerates are in the range of 0.3-3 μm. The morphology of the powder is characteristic of HEMM derived materials comprising brittle precursors.13 EDX mapping of the different elements was conducted to analyze the distribution of the species within the agglomerated particle. EDX analyses were subsequently conducted to analyze silicon and titanium in the powders containing 40 mol % Si  obtained after 20 h milling. The EDX analyses are shown in Fig. 4b and c, respectively. The bright spots correspond to the presence of each element, which appear to exist all over the particles. Based on the EDX elemental maps, both elements are distributed homogeneously within all particles, indicating that each particle contains a uniform distribution of Si and
obtained after 20 h milling. The EDX analyses are shown in Fig. 4b and c, respectively. The bright spots correspond to the presence of each element, which appear to exist all over the particles. Based on the EDX elemental maps, both elements are distributed homogeneously within all particles, indicating that each particle contains a uniform distribution of Si and 
Figure 4. (a) SEM micrograph of the  composite obtained after milling for 20 h. Chemical map of (b) Si and (c) Ti, respectively, using EDX. All images are taken at the same scale as shown in a.
composite obtained after milling for 20 h. Chemical map of (b) Si and (c) Ti, respectively, using EDX. All images are taken at the same scale as shown in a.
Due to the difficulty in measuring the size of the primary particles within the agglomerate using SEM, HRTEM was used to examine the primary particles of the  nanocomposite containing 40 mol % of Si obtained after milling for 20 h. Figure 5a and b shows the bright-field and dark-field images, respectively, of the nanocomposite. The agglomerated particle appears to be a very dense nanocomposite comprising nanosized crystallites. The dark-field image shows the nanocrystalline
nanocomposite containing 40 mol % of Si obtained after milling for 20 h. Figure 5a and b shows the bright-field and dark-field images, respectively, of the nanocomposite. The agglomerated particle appears to be a very dense nanocomposite comprising nanosized crystallites. The dark-field image shows the nanocrystalline  primary particles represented with bright spots, which are very small in the size range of 3-10 nm. Figure 5c shows a high magnification bright-field HRTEM image of the nanocomposites. The circular shape fringe, which is indicated with an arrow, corresponds to a
primary particles represented with bright spots, which are very small in the size range of 3-10 nm. Figure 5c shows a high magnification bright-field HRTEM image of the nanocomposites. The circular shape fringe, which is indicated with an arrow, corresponds to a 
 nanocrystallite.
nanocrystallite.
Figure 5. HRTEM micrographs of the electrode prepared with the  composite corresponding to
composite corresponding to  molar ratio obtained after milling for 20 h (a) bright-field, (b) dark-field, and (c) bright-field image taken at a higher magnification.
molar ratio obtained after milling for 20 h (a) bright-field, (b) dark-field, and (c) bright-field image taken at a higher magnification.
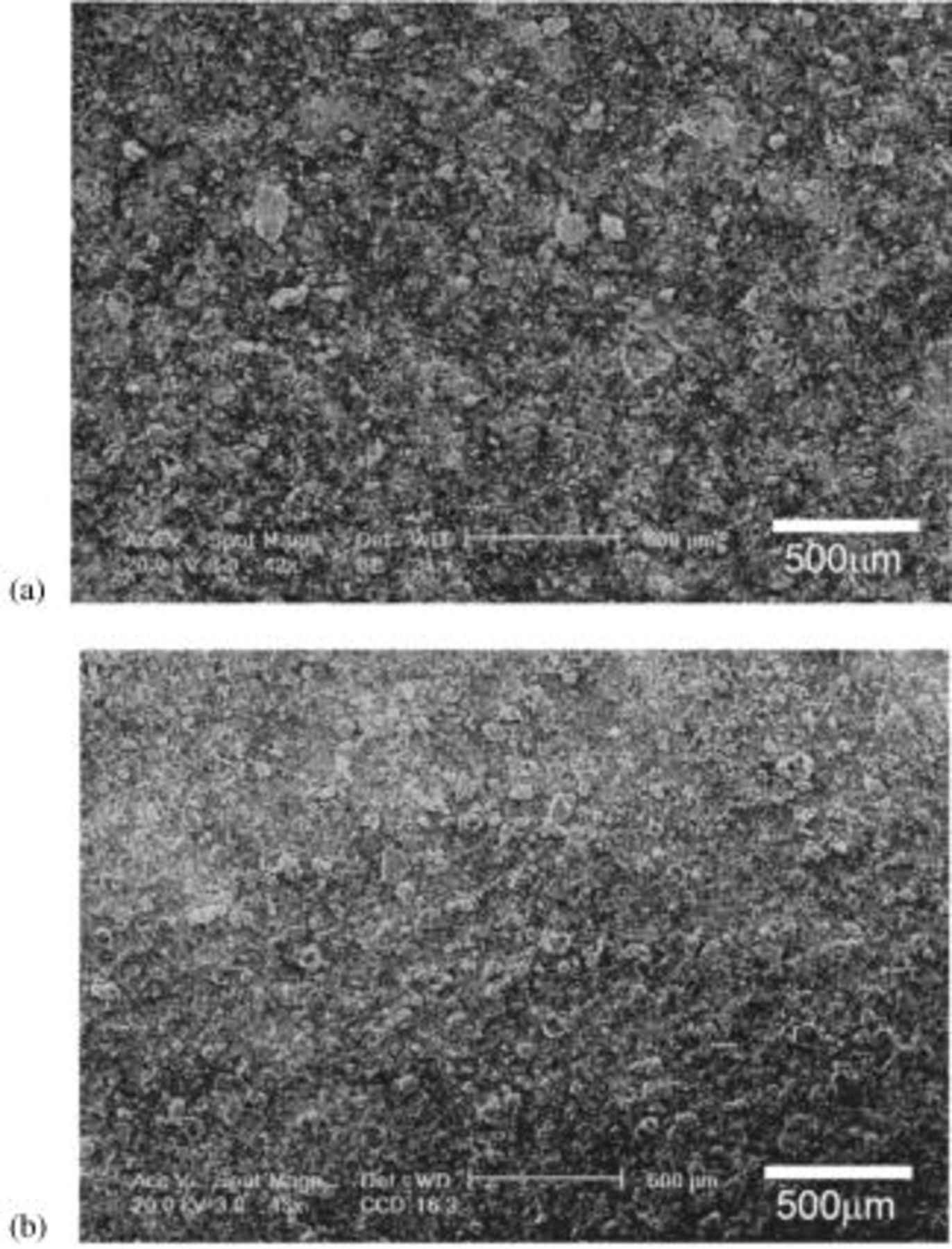
Generally, the Li-alloy based electrodes are known to exhibit structural damage such as cracking or crumbling after electrochemical cycling. The electrode prepared from the nanocomposite containing 40 mol % Si obtained after milling for 20 h has been analyzed using SEM to observe cracking if any, of the electrodes. Figure 6a is the SEM micrograph showing the surface of the electrode before cycling and Fig. 6b is the SEM image of the electrode after 20 cycles. No distinct change in morphology is observed before and after cycling, suggesting the good structural stability of this nanocomposite electrode, which is responsible for obtaining good capacity retention.
Figure 6. SEM micrographs of the electrode (a) before and (b) after 20 cycles, comprising  nanocomposite corresponding to
nanocomposite corresponding to  molar ratio obtained after milling for 20 h.
molar ratio obtained after milling for 20 h.
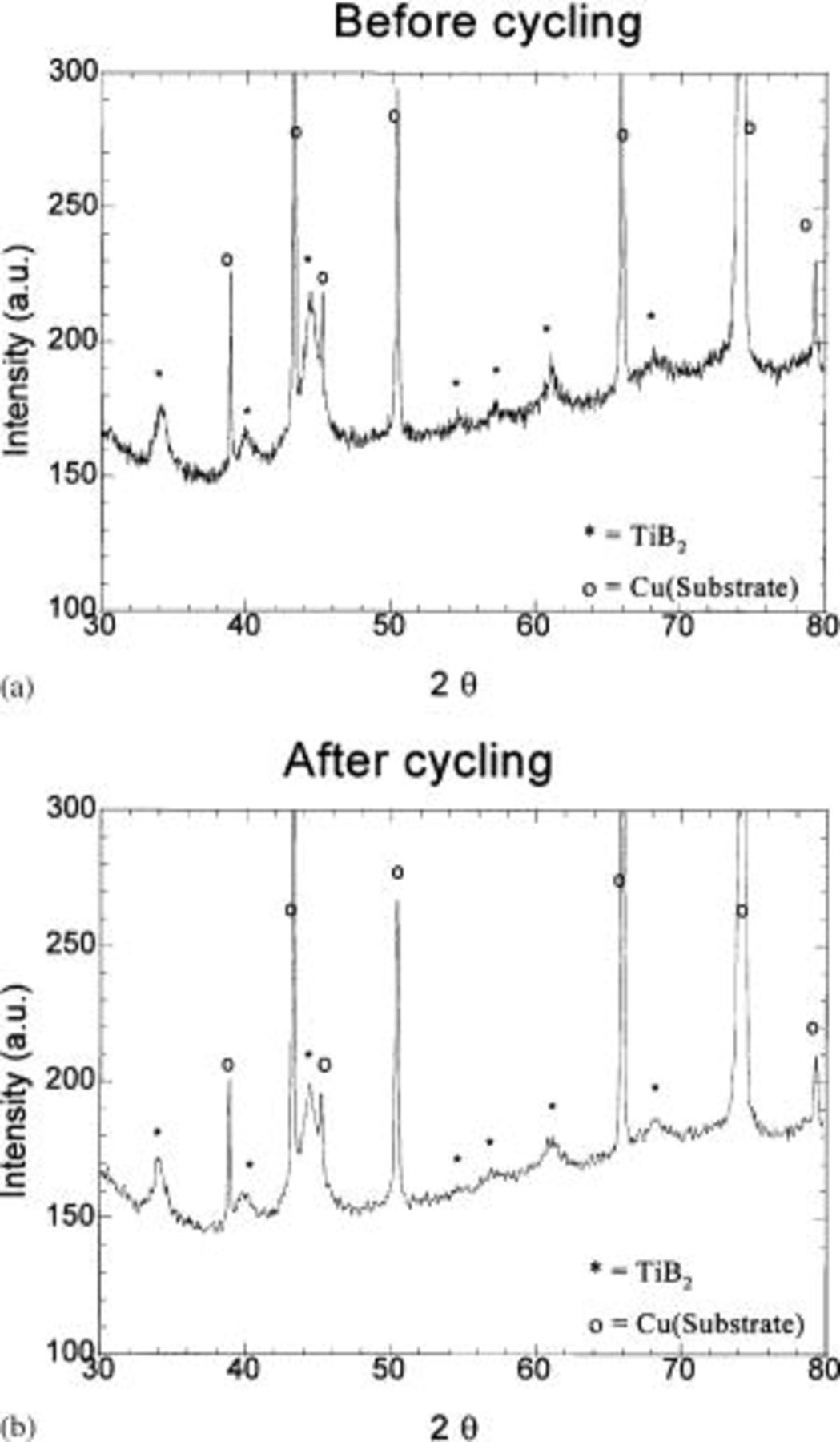
The electrode is also examined by XRD to verify the phase stability of the electrodes. Figure 7 shows the XRD spectra collected from the electrode before and after cycling. The sharp peaks in the XRD pattern correspond to the Cu substrate used for fabricating the electrode coatings. There is no observable change in the XRD patterns before and after electrochemical cycling, suggesting that Si reacts with lithium reversibly without undergoing any noticeable particle growth or secondary phase formation. This result suggests that the phase stability of the nanocomposite arises from the homogeneous distribution of the very fine amorphous active (Si) and nanocrystalline inactive  components.
components.
Figure 7. XRD pattern of the electrode before and after 20 cycles prepared with the composite corresponding to  molar ratio obtained after milling for 20 h.
molar ratio obtained after milling for 20 h.
To investigate the effect of initial particle size of  on the electrochemical properties of the anodes,
on the electrochemical properties of the anodes,  was premilled for different periods of time 12, 18, and 24 h, respectively. These premilled powders were used to generate the
was premilled for different periods of time 12, 18, and 24 h, respectively. These premilled powders were used to generate the  nanocomposites instead of commercial
nanocomposites instead of commercial  The Scherrer formula was used to determine the crystallite sizes of
The Scherrer formula was used to determine the crystallite sizes of  from the high-resolution X-ray plots collected on the premilled powders. The results are tabulated in Table I.
from the high-resolution X-ray plots collected on the premilled powders. The results are tabulated in Table I.
Table I.
Approximate crystallite size of the premilled 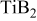 particles obtained after milling for 12, 18, and 20 h, respectively, calculated using the Scherrer formula. particles obtained after milling for 12, 18, and 20 h, respectively, calculated using the Scherrer formula. | |
|---|---|
| Milling time (h) | Approximate particle size(Å) |
| 12 | 71 |
| 18 | 57 |
| 24 | 56 |
Although the strain effect has not been considered for calculating the crystallite sizes due to the difficulties associated in measuring strain within the particles, the crystallite size nevertheless appears to decrease with increase in milling time. However, the decrease in crystallite size appears to be unaffected when milling is conducted for more than 18 h. The reason for this could be attributed to possible plastic deformation occurring in the particles when the crystallites are reduced to the size range of 57 Å. As a result, milling action now leads to deformation causing probable welding rather than shearing and pulverization of the brittle particles.
The  nanocomposite anodes obtained after milling commercial Si and premilled
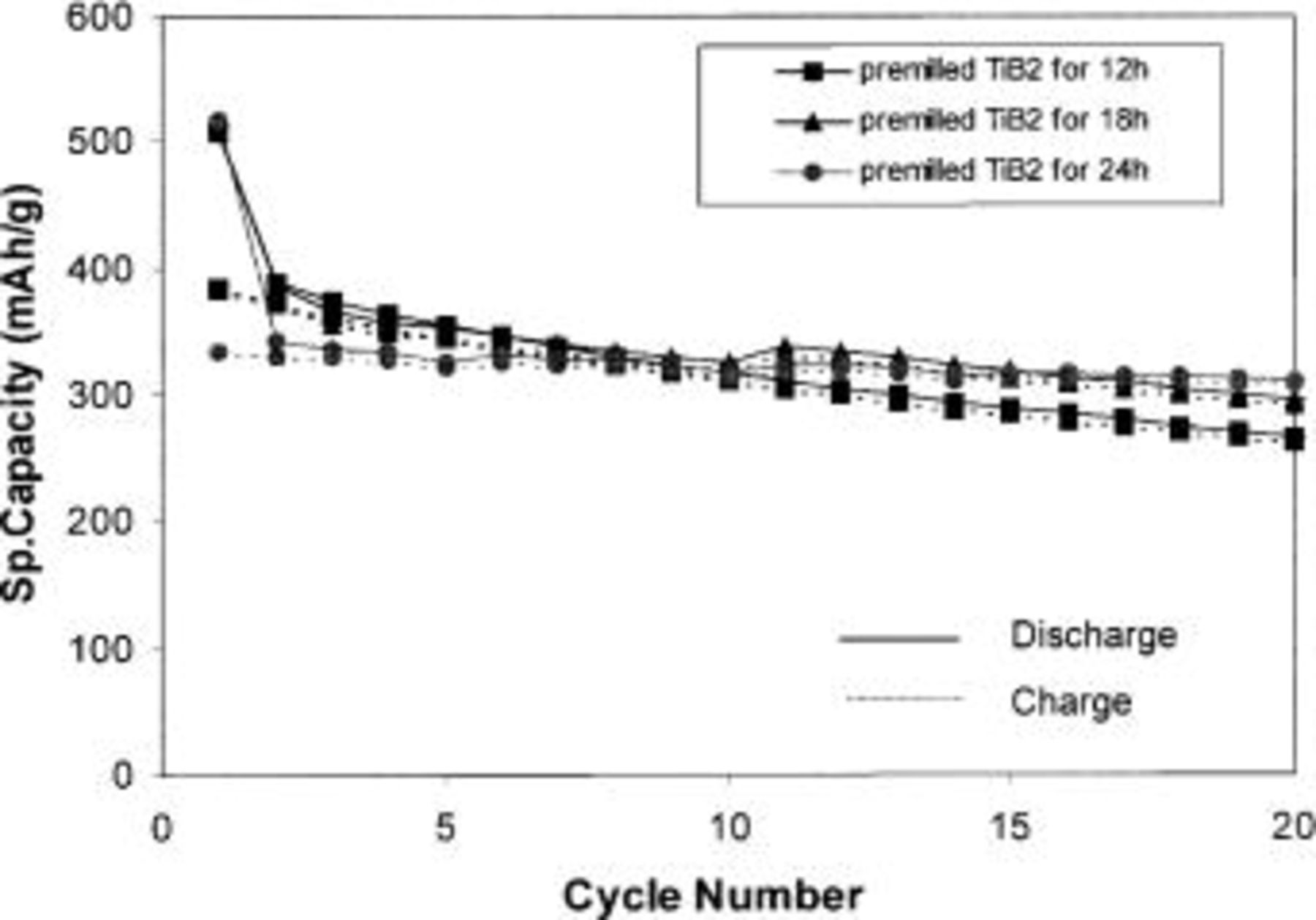
nanocomposite anodes obtained after milling commercial Si and premilled  powder mixtures for 10 h were then tested for electrochemical properties. The composition used for this study is the system containing 33 mol % rather than 40 mol % of the active phase because of the reduced milling time necessary to achieve relatively stable capacity. Furthermore, premilling of a larger amount of inactive component has a better effect on the capacity. As seen from the plot of capacity vs. cycle number (Fig. 8), the sample obtained using
powder mixtures for 10 h were then tested for electrochemical properties. The composition used for this study is the system containing 33 mol % rather than 40 mol % of the active phase because of the reduced milling time necessary to achieve relatively stable capacity. Furthermore, premilling of a larger amount of inactive component has a better effect on the capacity. As seen from the plot of capacity vs. cycle number (Fig. 8), the sample obtained using  premilled for 24 h shows better stability in terms of capacity retention (0.35% loss/cycle), which is normally observed in composites obtained after extended mechanical milling. The first discharge capacity is still lower than the theoretical capacity
premilled for 24 h shows better stability in terms of capacity retention (0.35% loss/cycle), which is normally observed in composites obtained after extended mechanical milling. The first discharge capacity is still lower than the theoretical capacity  as mentioned before. This result indicates that a small particle size of the inactive component provides better stability because the stress induced by volume expansion of the active phase during cycling is distributed more homogeneously in the composite containing premilled inactive component. This appears to be a good way to improve the electrochemical stability of the active-inactive nanocomposite anodes. The reduction in initial discharge capacity compared to the theoretical capacity and the overall reduction in capacity with prolonged milling are still issues that are being addressed currently, and will be presented in a subsequent publication.
as mentioned before. This result indicates that a small particle size of the inactive component provides better stability because the stress induced by volume expansion of the active phase during cycling is distributed more homogeneously in the composite containing premilled inactive component. This appears to be a good way to improve the electrochemical stability of the active-inactive nanocomposite anodes. The reduction in initial discharge capacity compared to the theoretical capacity and the overall reduction in capacity with prolonged milling are still issues that are being addressed currently, and will be presented in a subsequent publication.
Figure 8. Capacity as a function of cycle number for  nanocomposites with a molar ratio 1:2 obtained after milling for 10 h.
nanocomposites with a molar ratio 1:2 obtained after milling for 10 h.  used was premilled for 12, 18, and 24 h, respectively. Current rate:
used was premilled for 12, 18, and 24 h, respectively. Current rate:  potential: 0.02-1.2 V.
potential: 0.02-1.2 V.
Conclusion
The  nanocomposite composed of amorphous Si and nanocrystalline
nanocomposite composed of amorphous Si and nanocrystalline  can be obtained by milling Si and
can be obtained by milling Si and  for only 6 h. The
for only 6 h. The  nanocomposite corresponding to
nanocomposite corresponding to  obtained after milling for 20 h exhibits a stable gravimetric capacity as high as 400 mAh/g. The increase in milling time leads to a decrease in capacity of the nanocomposites because the amount of active silicon decreases as milling time increases. The nanocomposite obtained after milling for 20 h consists of agglomerates of nanosized particles, which are essentially nanocomposites of Si and
obtained after milling for 20 h exhibits a stable gravimetric capacity as high as 400 mAh/g. The increase in milling time leads to a decrease in capacity of the nanocomposites because the amount of active silicon decreases as milling time increases. The nanocomposite obtained after milling for 20 h consists of agglomerates of nanosized particles, which are essentially nanocomposites of Si and  as indicated by EDX and HRTEM results. Premilling of
as indicated by EDX and HRTEM results. Premilling of  increases the stability in capacity of the
increases the stability in capacity of the  nanocomposites, indicating that a small particle size of the inactive component provides better stability because the stress induced by volume expansion of the active phase during cycling is distributed more homogeneously in the composite containing the premilled inactive component.
nanocomposites, indicating that a small particle size of the inactive component provides better stability because the stress induced by volume expansion of the active phase during cycling is distributed more homogeneously in the composite containing the premilled inactive component.
Acknowledgments
The authors are grateful for the financial support of ONR (grant N00014-00-1-0516) and NSF (grant CTS-0000563).
Carnegie Mellon University assisted in meeting the publication costs of this article.