Abstract
Oxygen plasma resistance of low-k organosilica glasses (OSGs) is shown to be strongly dependent on the material structure: a silicon oxycarbide has a higher oxygen plasma resistance compared to a conventional OSG. The silicon oxycarbide is stable at pressures up to 300 mTorr, which is 10 times higher than those of the conventional OSG. Even at higher pressures, the degradation is much lower. Structural analysis using wet etching demonstrated that the stability at low pressures is due to a thin protecting layer: dense oxide formed by impingement of directional oxygen ions. The inside layer is shown to have the same k-value as the original film. The superior oxygen plasma resistance of the silicon oxycarbide is probably due to lower methyl group content, which provides greater volume reduction toward achieving a dense siloxane networks that protects the inside. © 2001 The Electrochemical Society. All rights reserved.
Export citation and abstract BibTeX RIS
Parasitic capacitance of on-chip multilevel interconnects has become a limiting factor in improving the operational speed and the power dissipation of ultralarge-scale integrated devices (ULSIs).1 To reduce the capacitance, various low permittivity (low-k) interlevel dielectrics (ILDs) have been investigated. Among them, organosilica glasses (OSGs), as well as aromatic polymers, are thought to be the most promising candidates.2 3 4 5
The OSGs typically consist of siloxane networks (Si-O-Si) and terminating methyl groups  as shown in Fig. 1a. The methyl groups are stable up to 650-700°C, which is sufficient for interconnect processing (400-450°C). The major concerns have been (i) mechanical strength, (ii) oxygen plasma resistance, and (iii) etching selectivity to stopper materials, like SiN.
as shown in Fig. 1a. The methyl groups are stable up to 650-700°C, which is sufficient for interconnect processing (400-450°C). The major concerns have been (i) mechanical strength, (ii) oxygen plasma resistance, and (iii) etching selectivity to stopper materials, like SiN.
Figure 1. Schematics of chemical bonding structures: (a) conventional OSG, and (b) silicon oxycarbide.
The low mechanical strength of OSGs is known to cause a delamination problem, for example, during Cu chemical mechanical polishing (CMP).3 The OSGs generally have lower mechanical strength than chemical vapor deposited (CVD) oxides because of the methyl groups terminating the networks. We have focused on this, and have improved the mechanical strength by modifying the bonding structure. This material, silicon oxycarbide  also contains methyl groups, but the number is estimated to be about 1/3-1/2 of that in conventional OSGs with
also contains methyl groups, but the number is estimated to be about 1/3-1/2 of that in conventional OSGs with  6
7 Instead, the carbon atoms play a part in networking, as shown in Fig. 1b. This network structure improves the mechanical strength to be over three times that of the conventional OSGs.
6
7 Instead, the carbon atoms play a part in networking, as shown in Fig. 1b. This network structure improves the mechanical strength to be over three times that of the conventional OSGs.
The weak oxygen plasma resistance of the OSGs have been known to cause a via connection failure, called via poisoning. Via poisoning was first reported as a failure in Al interconnects using the OSGs as ILDs.8 The failure occurs when the OSGs are exposed on via sidewalls and are directly subjected to oxygen plasma for ashing. The oxygen plasma converts the OSGs into porous oxides absorbing much moisture. This moisture is released during subsequent metal deposition and prevents via connections. In a Cu damascene process, the oxidation also degrades the effective k and increases the leakage current between adjacent interconnects, since the OSGs are also exposed on interconnect sidewalls and subjected to the oxygen plasma. This is a more severe problem than via poisoning, however, because the dielectric properties are very sensitive to moisture absorption. Employing low pressure  -reactive ion etching (RIE) has been reported to be effective to suppress porous oxidation.2
9 However, the mechanism of this has not been clarified. Furthermore, the material properties essential to this effect have not been clarified, thus obscuring studies of the effectiveness of other OSGs, which are different in carbon contents, density, and other properties.
-reactive ion etching (RIE) has been reported to be effective to suppress porous oxidation.2
9 However, the mechanism of this has not been clarified. Furthermore, the material properties essential to this effect have not been clarified, thus obscuring studies of the effectiveness of other OSGs, which are different in carbon contents, density, and other properties.
In this paper, we report the resistance of different OSG materials against oxygen plasma with different pressures. A silicon oxycarbide is shown to have much higher oxygen plasma resistance than a conventional OSG. Structural analysis of the oxidized films clarifies the suppression mechanism of porous oxidation at low pressures. Desirable material properties to ensure the effect for all patterns regardless of size, density, and aspect ratio, are discussed.
Experimental
Blanket silicon oxycarbide films  were prepared by plasma-enhanced (PE) CVD using methyltriethoxysilane (MTES) and nonoxidizing gas in a parallel plate-type chamber.6
7 The typical deposition condition was as follows: the pressure was 5 Torr, and the radio frequency (rf) power was 1.6 W/cm2, and the substrate temperature was 375°C. A conventional OSG
were prepared by plasma-enhanced (PE) CVD using methyltriethoxysilane (MTES) and nonoxidizing gas in a parallel plate-type chamber.6
7 The typical deposition condition was as follows: the pressure was 5 Torr, and the radio frequency (rf) power was 1.6 W/cm2, and the substrate temperature was 375°C. A conventional OSG  and an oxide [plasma-enhanced tetraethylorthosilicate (PTEOS)
and an oxide [plasma-enhanced tetraethylorthosilicate (PTEOS)  ] were also prepared as references.
] were also prepared as references.
The oxygen plasma treatment was carried out by RIE using a Tokyo Ohka TCE-4802. The pressure was 10 to 500 mTorr, the rf power was 0.8-W/cm2, and the treatment time was 1 min. A barrel-type asher, an IPC Reactor Center, was also used for comparison. The pressure was 1 Torr, the rf power was 0.03 W/cm3, and the treatment time was 15 min.
To evaluate the degradation, the thickness and the k were primarily monitored. The thickness was measured using a Gaertner L115A ellipsometer. The k was extracted from a capacitance-voltage (C-V) measurement. An HP-4275A LRC meter was employed for the C-V measurements.
The vertical structure of an oxidized film was analyzed using repetitions of wet etching and measurements by ellipsometory and Fourier transform infrared spectroscopy (FTIR). Dilute 0.5% HF solution was used for the wet etching. A Bio-Rad QS-300 spectrometer was used for the FTIR.
Results and Discussion
Figure 2 compares the changes in k of the silicon oxycarbide and a conventional OSG during  RIE for 1 min. The x axis represents the pressure of the RIE. The initial sample thickness was 200 nm for the silicon oxycarbide
RIE for 1 min. The x axis represents the pressure of the RIE. The initial sample thickness was 200 nm for the silicon oxycarbide  and 250 nm for the conventional OSG
and 250 nm for the conventional OSG  The conventional OSG showed significant change at high pressures as follows: up to a pressure of 30 mTorr, the k was as low as 3.1. The k increased to 4.0 at 100 mTorr and exceeded 16 at higher pressures. This extremely high k was accompanied by high leakage current, since the tangent delta during the C-V measurements was significantly large: over 0.1. In contrast, the change of silicon oxycarbide was much more mild. At pressures up to 300 mTorr, the k was almost constant: about 3.9. At 800 mTorr, the k increased to 5, which is much lower than that of the conventional OSG. The transition pressure at which the k increased was 10 times higher than that of the OSG. This clearly shows that the oxygen plasma resistance of the silicon oxycarbide is higher than that of the OSG.
The conventional OSG showed significant change at high pressures as follows: up to a pressure of 30 mTorr, the k was as low as 3.1. The k increased to 4.0 at 100 mTorr and exceeded 16 at higher pressures. This extremely high k was accompanied by high leakage current, since the tangent delta during the C-V measurements was significantly large: over 0.1. In contrast, the change of silicon oxycarbide was much more mild. At pressures up to 300 mTorr, the k was almost constant: about 3.9. At 800 mTorr, the k increased to 5, which is much lower than that of the conventional OSG. The transition pressure at which the k increased was 10 times higher than that of the OSG. This clearly shows that the oxygen plasma resistance of the silicon oxycarbide is higher than that of the OSG.
Figure 2. Permittivity (k) change after  RIE for 1 min.
RIE for 1 min.
As for the thickness, similar results were obtained as shown in Fig. 3. The thickness of the conventional OSG reduced by about 10 nm at pressures up to 30 mTorr. The reduction increased to 40 nm at 100 mTorr, and to over 80 nm at higher pressures. In contrast, the thickness reduction of the silicon oxycarbide was only 0.1 nm at 10 mTorr, and was less than 10 nm at pressures up to 300 mTorr. Even at 800 mTorr, the reduction was 14 nm, which is less than 20% of that of the conventional OSG.
Figure 3. Thickness reduction after  RIE for 1 min.
RIE for 1 min.
The oxidized conventional OSGs were analyzed using wet etching in dilute HF, as shown in Fig. 4. The conventional OSG treated at 10 mTorr was initially hydrophilic, and the etching rate was almost constant: 24 nm/min. When the 12 nm thick layer was removed, the surface changed to be hydrophobic, and the etching rate decreased sharply. This is because the unoxidized OSG surface was exposed. The 12 nm thick, hydrophilic layer was identified to be an oxide from the subtracted FTIR spectrum shown in Fig. 5. This oxide is dense, because the etching rate is comparable to that of PTEOS: 11 nm/min. In the case of 100 mTorr, bilayer oxides covered the OSG. The surface layer was also a dense oxide, but was only 5 nm thick. The intermediate layer was a 56 nm thick, porous oxide with etch rate of 160 nm/min, which is 15 times higher than that of the PTEOS. This extremely high etching rate is almost the same as that of the conventional OSG porously oxidized at 1 Torr using the barrel-type asher: 216 nm/min. This demonstrates that suppression of porous oxidation at low pressures is due to formation of a dense oxide layer, which protects the inside against oxygen radical penetration. The density is comparable with that of PTEOS. This increase in oxide density is due to impingement of directional oxygen ions, since it arises only at low pressures.
Figure 4. Vertical profile of oxidized OSG analyzed by wet etching. Wet etching solution is 0.5% dilute HF.
Figure 5. FTIR spectrum of thin, surface oxidized layer.
The oxidized silicon oxycarbide films were also analyzed in the same way, as shown in Fig. 6. There was little difference between 10 and 100 mTorr cases. The surface layers were dense oxides with etching rate of 24 nm/min, which was almost the same as that of the conventional OSGs. The thickness was 14 and 19 nm for 10 and 100 mTorr, respectively. There was no porous layer even at 100 mTorr. We also measured the etching rate after porous oxidation using the barrel-type asher. The etching rate was 126 nm/min, which is just over half of that of the conventional OSG: 216 nm/min. This clearly shows that the oxidized silicon oxycarbide is denser than the oxidized conventional OSG in the cases without directional oxygen ions.
Figure 6. Vertical profile of oxidized silicon oxycarbide.
To investigate the inside layer protected by the dense surface, the k of the silicon oxycarbide was measured after wet etching using dilute HF, as shown in Fig. 7. Before the wet etching, the k was about 3.8. After the wet etching, however, the k reduced to about 3.4, which is almost the same value as the original films. This clearly shows that the film inside is not degraded during the  RIE. In practical fabrication process, this surface layer can be removed during sputter etching prior to the metal deposition.
RIE. In practical fabrication process, this surface layer can be removed during sputter etching prior to the metal deposition.
Figure 7. Change in k by removing the surface oxidized layer. The samples were the silicon oxycarbide.
The material difference is discussed from the viewpoint of bonding structures. The methyl groups in the conventional OSG are oxidized in oxygen plasma as follows

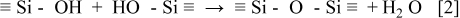
The initial siloxane networks are porous due to terminating methyl groups. In addition, the Si-C bond length is 0.19 nm, which is almost the same as Si-O bond length in siloxane networks: 0.18 nm. This means that much volume reduction is needed to achieve dense siloxane networks. On the other hand, the Si-C-Si bonds in the silicon oxycarbide are oxidized as follows
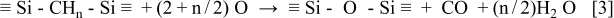
The volume change after the oxidation should be negligible. In other words, the Si-C-Si bonds are originally almost as dense as the siloxane bonds. The silicon oxycarbide also contains methyl groups, but the number is estimated to be about 1/3-1/2 of that in conventional OSGs with  6
7 This means that the total volume reduction to achieve dense siloxane networks on silicon oxicarbide surface is much less than that of the conventional OSGs. This is probably the reason for higher transient pressure and less degradation of the silicon oxycarbide.
6
7 This means that the total volume reduction to achieve dense siloxane networks on silicon oxicarbide surface is much less than that of the conventional OSGs. This is probably the reason for higher transient pressure and less degradation of the silicon oxycarbide.
The analysis here is based on the blanket film experiments. In real situations, the oxygen plasma is applied to remove the photo resist after via and/or trench patterning. In the case of via and/or trench sidewalls, the transient pressure should be effectively higher because the number of impinging ions and the energy transfer from the ions are much less than those in the blanket films. Increasing rf power to compensate for this may increase the sputter etching effect during the  RIE. If the thickness reduction and the faceting due to the sputter etching are significant, the OSG surface should be capped with a harder material, for example, like an oxide. Using such a stacked structure, however, increases the effective k. For example, if the material with
RIE. If the thickness reduction and the faceting due to the sputter etching are significant, the OSG surface should be capped with a harder material, for example, like an oxide. Using such a stacked structure, however, increases the effective k. For example, if the material with  needs to be capped, the effective k can be higher than that of material with
needs to be capped, the effective k can be higher than that of material with  without using a cap.6
7 Materials with higher transient pressure, like silicon oxycarbide, are desirable for this reason.
without using a cap.6
7 Materials with higher transient pressure, like silicon oxycarbide, are desirable for this reason.
Conclusions
Oxygen plasma resistance of low k OSGs was found to be strongly dependent on the material structure: a silicon oxycarbide has superior oxygen plasma resistance than a conventional OSG. The k and thickness of the blanket silicon oxycarbide film were stable at pressures up to 300 mTorr, which is 10 times higher than those of the conventional OSG. Even at higher pressures, the degradation of the silicon oxycarbide was much lower. Structural analysis using wet etching demonstrated that the stability at low-pressures is due to a thin protecting layer: 14 to 19 nm thick, dense oxide formed by impingement of directional oxygen ions. The inside layer was shown to have the same k value as the original film. The superior oxygen plasma resistance of the silicon oxycarbide is probably due to lower content of methyl group. This is because less volume reduction is needed to achieve a dense siloxane network protecting the inside.
Acknowledgments
The authors give thanks to H. Morishima in Hitachi Chemical Co., Ltd., A. Maekawa, in Hitachi ULSI Engineering Co., Ltd, and N. Ohashi and N. Owada (currently in Applied Materials, Japan) in Device Development Center, Hitachi Ltd., for useful discussions.
Hitachi, Limited assisted in meeting the publication costs of this article.








