Abstract
There are an increasing number of studies regarding active electrode materials that undergo faradaic reactions but are used for electrochemical capacitor applications. Unfortunately, some of these materials are described as "pseudocapacitive" materials despite the fact that their electrochemical signature (e.g., cyclic voltammogram and charge/discharge curve) is analogous to that of a "battery" material, as commonly observed for Ni(OH)2 and cobalt oxides in KOH electrolyte. Conversely, true pseudocapacitive electrode materials such as MnO2 display electrochemical behavior typical of that observed for a capacitive carbon electrode. The difference between these two classes of materials will be explained, and we demonstrate why it is inappropriate to describe nickel oxide or hydroxide and cobalt oxide/hydroxide as pseudocapacitive electrode materials.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
In the field of electrochemical capacitors (ECs, also known as "supercapacitors") the term "pseudocapacitance"1 is used to describe the behavior of electrode materials (RuO2,2,3 MnO2,4,5 ...) that have the electrochemical signature of a capacitive electrode, i.e., a linear dependence of the charge stored with changing potential within the window of interest, but in which charge storage originates from electron-transfer mechanisms, rather than simply relying on the accumulation of ions in the electrochemical double layer (as with activated carbons). Despite this clear definition, many battery-type electrodes such as Ni(OH)26,7 or other materials that exhibit faradaic behavior (even those that are electrochemically irreversible)8,9 have been presented in the literature as pseudocapacitive materials, which leads to confusion for readers because the concept of "capacitance" (F) cannot apply to purely faradaic behavior, where "capacity" (coulomb, C, or mAh) is the most appropriate and meaningful metric to use in such cases.
In this communication, we will discuss the fundamental electrochemistry behind true pseudocapacitance mechanisms and provide guidance with respect to how such descriptions should be used (or not used) when reporting on new materials for ECs.
Some Definitions
The origin of the word "pseudocapacitance" can be found in the association of the prefix "pseudo" and capacitance. In the dictionary,10 the term "pseudo" (from the Greek combining form of pseud s false, pse
s false, pse dos falsehood) has two meanings. It can be used to describe something that is false or fake, deceptive, a sham (example: pseudo-science, a theory, methodology, or practice that is considered to be without scientific foundation). A second meaning is: (i) not actually but having the appearance of; (ii) almost, approaching, or trying to be (example: pseudo-classicism, the imitative use of classicism in art and literature, prevalent during the 18th century). Because the term "pseudocapacitance" was created in order to describe the properties of an electrode that behaves like a capacitor in its electrochemical signature, this second definition of "pseudo" is the more correct use in the present case.
dos falsehood) has two meanings. It can be used to describe something that is false or fake, deceptive, a sham (example: pseudo-science, a theory, methodology, or practice that is considered to be without scientific foundation). A second meaning is: (i) not actually but having the appearance of; (ii) almost, approaching, or trying to be (example: pseudo-classicism, the imitative use of classicism in art and literature, prevalent during the 18th century). Because the term "pseudocapacitance" was created in order to describe the properties of an electrode that behaves like a capacitor in its electrochemical signature, this second definition of "pseudo" is the more correct use in the present case.
Capacitance is the ability of a body to store an electrical charge. This capacitance is constant over a given voltage window and can be used to calculate the charge stored using equation 1,
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/162/5/A5185/revision1/jes_162_5_A5185eqn1.jpg)
where ΔQ is the charge stored (expressed in coulombs, C, or mAh) and ΔU is the width of the voltage window (V). In this case the capacitance, C, is the amount of charge stored when a 1 V window is used. For a given voltage window there is a direct and simple access to the charge stored.
In the field of electrochemical capacitors, the term "pseudocapacitance" is used to designate electrode materials (RuO2, MnO2) that have the electrochemical signature of a capacitive electrode (such as observed with activated carbon), i.e., exhibiting a linear dependence of the charge stored with the width of the potential window, but where charge storage originates from different reaction mechanisms.2,3,4,5 This definition can be found in Conway's influential book, "Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications.1 Conway stated that: "Regular double layer capacitance arises from the potential-dependence of the surface density of charges stored electrostatically (i.e., non-faradaically) at the interfaces of the capacitor electrodes. (...) Pseudocapacitance arises at the electrode surfaces where a completely different charge-storage mechanism applies. It is faradaic in origin, involving the passage of charge across the double layer, as in battery charging or discharging, but capacitance arises in account of the special relation that can originate for thermodynamic reasons between the extent of charge acceptance (Δq) and the change of potential (ΔV), so that the derivative d(Δq)/d(ΔV) or dq/dV, which is equivalent to a capacitance, can be formulated and experimentally measured by dc, ac, or transient techniques. (...)."
Two-Terminal Devices
Electrochemical energy-storage devices can readily exhibit two extreme types of behavior when charged/discharged under constant current. The first one is related to batteries (either primary or secondary batteries) and most often results in a flat discharge plateau. The second one is related to a capacitor and exhibits a triangular-shaped plot upon cycling. Intermediate behavior can be found when a faradaic electrode exhibits a sloped discharge curve due to the presence of a solid solution, as observed for LiCoO2.11
However, the macroscopic behavior of a given device does not presume the processes that occur at each electrode inside the device, and furthermore does not indicate whether individual electrodes are capacitive or faradaic in nature. So-called asymmetric or hybrid devices, for example, are assembled with a battery-type negative (graphite, Li4Ti5O12, TiO2(B),...)12,13,14 or positive electrode (PbO2, Ni(OH)2, ...)15,16 and a complementary capacitive electrode, typically activated carbon. The resulting charge/discharge plot of the device looks capacitive because it is the combination of a capacitive electrode (triangular shape) and a faradaic one (plateau shape). Thus, it is not possible to use the term pseudocapacitive for a two-terminal device, but it should only be used for a given electrode investigated individually in a three-electrode cell as described later on.
Discussion
Activated carbon/Ni(OH)2 hybrid (asymmetric) device
Electrochemical capacitors that are based on activated carbons and double-layer capacitance mechanisms (i.e., electrical double-layer capacitors, EDLCs) exhibit technologically relevant performance when operated in nonaqueous electrolytes, but have limited energy density with aqueous electrolytes because of lower operating voltages (rarely exceeding 1 V),17 and stability issues related to carbon oxidation.1,15,16,18 Consequently, each carbon electrode has to work in a limited electrochemical window of ≈0.5 V, which means that the resulting capacitance (F g−1) of the carbon symmetrical device is only one fourth of that of a single carbon electrode when measured in a three-electrode cell.15
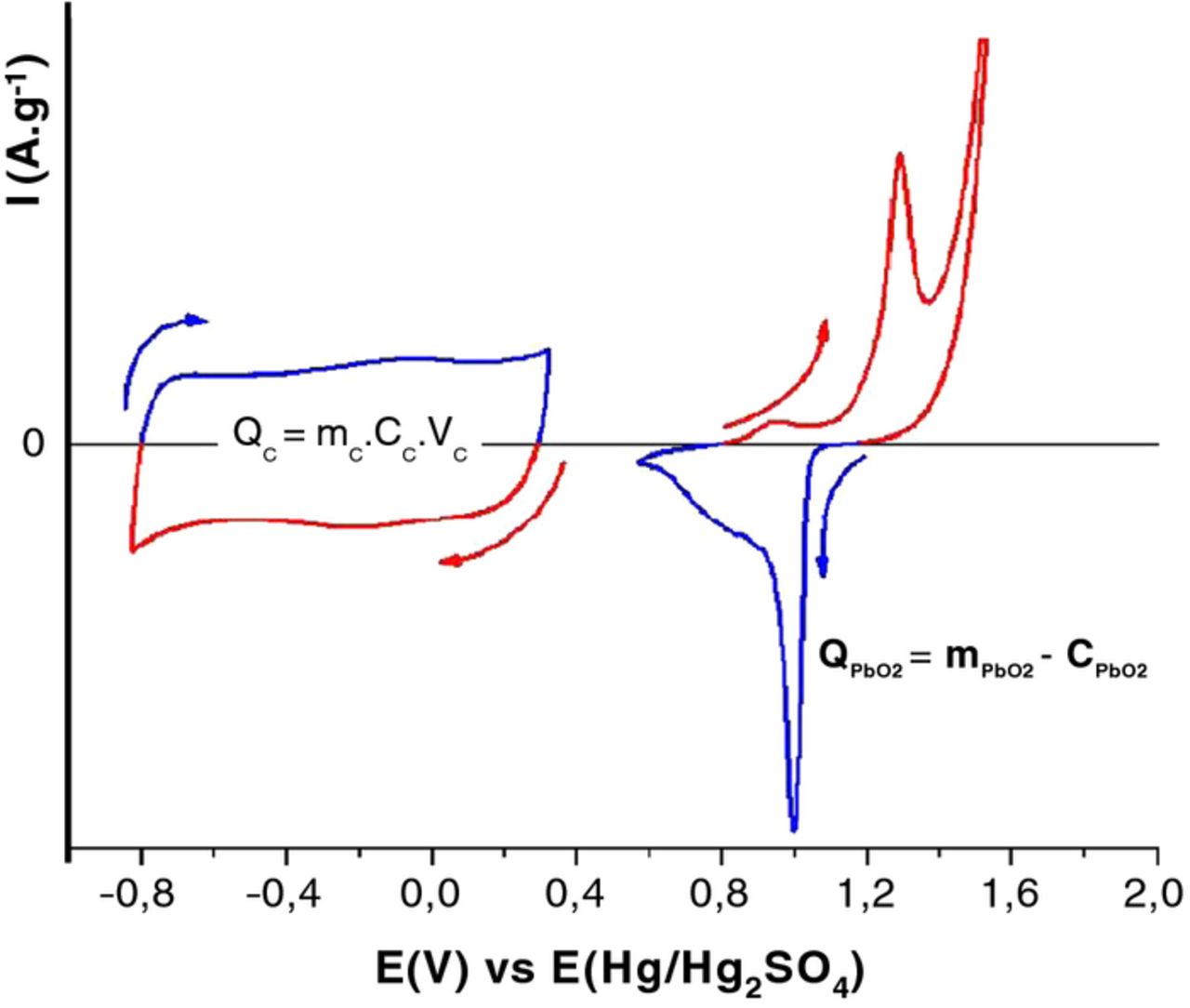
One way to circumvent such limitations is to design an asymmetric (or hybrid) device that combines an activated carbon negative electrode19 with a battery-type positive electrode, typically PbO215 or Ni(OH)2,16 that is faradaic in nature and operated in a complementary electrochemical window (Fig. 1).
Figure 1. Activated carbon/PbO2 hybrid (asymmetric) device in 1M H2SO4. mc and mPbO2 are respectively the mass of carbon and PbO2, Cc is the gravimetric capacitance (in F.g−1) of carbon electrode, CPbO2 is the gravimetric capacity (in C.g−1 or mAh.g−1) of the lead dioxide electrode, Vc is the width of the electrochemical window in which the carbon electrode is cycled.
As a result of this asymmetric electrode pairing, cell voltage in aqueous electrolytes is usually increased above 1 V, and the carbon electrode can be operated in its full electrochemical window. It should be noted that the specific capacity (in C g−1) of the carbon is usually much less than that of the faradaic positive electrode. With increased operating voltage and the use of high-capacity faradaic electrodes, the energy density of such hybrid devices is higher than that of carbon symmetrical ECs.
The first report of an energy-storage device that combined an electric double layer capacitor electrode with a positive nickel battery electrode (a so-called hybrid electrochemical capacitor) was a patent by Varakin et al. in the mid 90's.20 The patent states: "The essence of the invention resides in that used in the same capacitor are electrodes made of different materials, that is, one of the electrodes is made of a fibrous carbonic material (as in conventional double-layer capacitors), whereas the other capacitor is a nickel-oxide one. It is due to such a combination of electrodes that the inventors were managed quite unexpectedly to improve many times the principal characteristics of the capacitors. Thus, the specific capacity of the capacitor increases 8–10 times."
A second patent of note21 that relates to the ratio of electrode capacity in a hybrid electrochemical capacitor provides the definition for an asymmetric electrochemical capacitor. In essence, capacitor-like cycle life is achieved by limiting the faradaic electrode's depth of discharge. Several manufacturers specify >100,000 cycles for their asymmetric capacitors).22,23 Further, device operating voltage (thus energy density) is increased because the electrodes have dissimilar materials.
It seems now well-established that the term "hybrid" supercapacitor should be used when pairing two electrode with different charge-storage behavior, i.e., one capacitive and one faradaic, and the resulting device is in-between a supercapacitor and a battery. "Asymmetric" supercapacitor covers a wider range of electrode combinations because it can be used for supercapacitors using electrodes of the same nature but with different mass loading, or two electrodes using different materials. We suggest the term "asymmetric" should be used only when capacitive or pseudocapacitive electrodes are involved (such as activated carbon//MnO2 asymmetric supercapacitor)24,25,26 in order to avoid confusion with true "hybrid" devices.
For hybrid devices, full calculations of projected energy densities can be found in the work of Zheng and Conway.15,16 A maximum energy density of 50 Wh.kg−1 is estimated for an activated carbon/Ni(OH)2 hybrid device based on a 1.65-V working voltage in 6.25 M KOH and a positive/negative electrode weight ratio equals to 1/3. For comparison, asymmetrical activated carbon EDLC in 5.26 M H2SO4 exhibits only 7.2 Wh kg−1.16 The main drawbacks of the hybrid device are its moderate power density and cycle life, both limited by the positive Ni(OH)2 electrode. Thus, most work on the activated carbon/Ni(OH)2 device targets the improvement of rate capability and cycling stability at the positive faradaic electrode.
In alkaline electrolyte, the theoretical maximum capacity associated to the following one-electron reaction (eq. 2) is 290 mAh g−1:
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/162/5/A5185/revision1/jes_162_5_A5185eqn2.jpg)
Although a seemingly straightforward reaction, the Ni(OH)2 electrode undergoes more complicated processes during cycling, ultimately limiting its rate capability. To circumvent power/rate limitations, recent research has focused on: (i) new synthesis routes of Ni(OH)2 nanoparticles;27 and (ii) fabrication of composite materials comprising Ni(OH)2 particles that are incorporated with a conductive carbonaceous material such as activated carbon28 nanotubes or graphene sheets29,30 to improve electronic conductivity within the electrode. Such strategies are chosen to maintain the high specific capacity that is characteristic of Ni(OH)2 while improving electron transfer, thus increasing the power density of the resulting hybrid device. Many advances have been reported for Ni(OH)2 in recent years, but unfortunately their impact has been mitigated by a confusion of nomenclature that was introduced to the scientific community working in that field at its early stages, whereby the Ni(OH)2 electrode was described as "pseudocapacitive" rather than faradaic (battery-type electrode).6 This mislabeling of the charge-storage mechanism has since propagated through the literature on Ni(OH)2 materials proposed for EC applications.
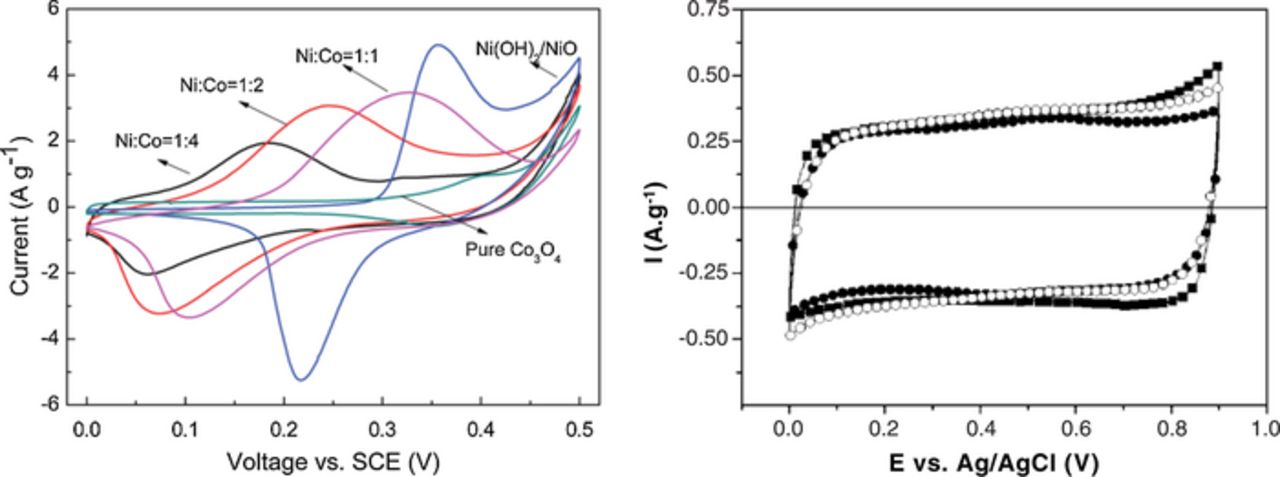
For example in the paper of Hu et al.,6 the authors claimed that "A pair of redox peaks can be observed in each CV curves which indicates a pseudocapacitance characteristic". Indeed redox waves are clearly observed in the CVs (Fig. 2a), features that are typical of faradaic battery-type electrodes, and certainly distinct in behavior from a true pseudocapacitive electrode.
Ni(OH)2 vs MnO2
Conway's definition allows one to differentiate faradaic electrodes from pseudocapacitive ones. In Fig. 2a, integrating the oxidative sweep of the CV for Ni(OH)2 from 0.0 to 0.3 V vs SCE leads to a capacity that is negligible (almost 0 C g−1 because it is the double-layer capacitance of Ni(OH)2), which obviously translates to a capacitance close to 0 F g−1.6 If the same calculation is performed in a different potential window (0.3 to 0.4 V vs SCE), the capacity is close to 300 C g−1 and a capacitance of 3000 F g−1 can be calculated (which is not correct). If this capacitance is calculated between 0.4 to 0.5 V vs SCE it decreases down to 2000 F g−1. These calculations clearly show that the "capacitance" for Ni(OH)2 electrode is definitely not constant over the whole potential window available. Consequently, Ni(OH)2 electrodes cannot be considered as pseudocapacitive, in contradiction with the authors who state so.
Very different behavior is observed for RuO2 or MnO2 electrodes. Fig. 2b shows the CV of an amorphous MnO2 electrode.5 In this case, a constant capacitance of 150 F g−1 can be calculated throughout the whole potential window. In this figure, the CV of MnO2 (pseudocapacitive electrode) clearly resembles that of activated carbon (capacitive electrode), in accordance with the definition of the prefix, pseudo (1. not actually but having the appearance of; 2. almost, approaching, or trying to be.). In the case of carbon electrodes, their electrochemical signature is mainly attributed to double-layer capacitance arising from the potential-dependence of the surface density of charges stored electrostatically (i.e., non-faradaically) at the interfaces of the capacitor electrodes. In the case of MnO2 electrodes, a similar signature arises from reactions that are faradaic in origin, involving the passage of charge across the double layer. This pseudocapacitance is due to fast and reversible faradaic reactions that have been demonstrated in many studies, and which involve the electrochemical interconversion between Mn4+and Mn3+ in the solid, and corresponding insertion of cations, H+ or C+ (C+ = Na+, K+, ...), from the electrolyte.31,32,33,34 The same argument applies to the RuO2 electrode where proton insertion is the main cause of pseudocapacitance, concomitant with a change in Ru oxidation state over the potential window.35,36
There are now more than 300 papers that describe Ni-based electrodes as pseudocapacitive, which, as presented above, does not follow the intended definition of the pseudocapacitance term. The possible origin of this confusion lies in the electrical signature of the full two-electrode device, which is usually investigated without the use of a third reference electrode. This kind of two-terminal device does exhibit a capacitor-like behavior from a purely from an electrical point of view, but definitely not from an electrochemical one.37 The sloping discharge curve for these asymmetric devices is primarily because the negative electrode is in fact a capacitive electrode.
The above mentioned confusion also clearly demonstrates that pseudocapacitive terminology cannot be applied to a full device but must be used to describe the electrochemical behavior of a specific material processed as an electrode.
Capacity vs capacitance
Park et al.,38 stated that "In order to enhance energy density, a hybrid type pseudocapacitor/electric double layer capacitor (EDLC) was considered and its electrochemical properties were investigated. At various current densities, stable charge/discharge behaviors were observed with much higher specific capacitance values of 530 F g−1 compared with that of EDLC (230 F g−1), by introducing Ni(OH)2 as a cathode material." These two values of capacitance cannot be compared because 530 F g−1 corresponds to an "average" value calculated over a limited potential window, but, as demonstrated in the former paragraph, is not constant, and if a wider or narrower potential window is chosen, the calculated specific capacitance will change. In this case, only the capacity in C g−1 or mAh g−1 provides a consistent metric with which to compare against other materials. Comparison with the carbon electrode can be made by translating the constant capacitance of the carbon electrode into C g−1 using the width of the potential window that is traversed when cycled in the device. Thus, the total charge stored at each electrode can be directly compared. Similar treatment can be applied to a MnO2 pseudocapacitive electrode, which also exhibits a rectangular-shape CV. The signature of a faradaic electrode such as Ni(OH)2 is totally different.
Unfortunately, there are situations where the electrochemical behavior of a faradaic electrode can appear pseudocapacitive. This can be the case when the electrode material (powder, thin film, ...) reaches a critical size39,40 where diffusion occurs through very limited timescales, giving rise to a "capacitive like" behavior. But in such cases capacitor-like behavior is only due to the electrode design or architecture and not an intrinsic property of the investigated material, as observed with LiCoO2 thin films (6-nm thick).40 LiCoO2 is known as a lithium-intercalation compound used as positive electrode in lithium-ion batteries and does not show "pseudocapacitive" behavior in such devices. Thus, only the appropriate material engineering causes the pseudocapacitive property to emerge, unlike MnO2 in mild aqueous electrolyte, which shows pseudocapacitive behavior regardless of the electrode design. In the case of LiCoO2, Dunn et al. denoted that such behavior is "extrinsic" pseudocapacitance, while in the case of MnO2 capacitor-like behavior is intrinsic to the material.40 It is worth mentioning that in this case also the authors are using this term for a single electrode and not for a device.
Cobalt-based electrodes
The same confusion between faradaic and pseudocapacitive electrodes can also be found for cobalt-based oxides or hydroxides,41 or even nickel-cobalt oxides or hydroxides (Figure 3).42 Additionally, some papers describing these compounds attempt to make reference to earliest work of Conway.43
Figure 3. Cyclic voltammograms (10 mV.s−1) of an electroplated Co3O4 thin film annealed at 300°C, using different upper potential limits, in 1 M KOH electrolyte.
Conway stated that: "Although similar films arise at Ru electrodes, the development of distinguishable reversible states is obscured in that case by overlap between the responses of several (? three) oxidation states, giving rise to an almost rectangular current/voltage profile44 approaching that for an ideal capacitor as discussed in Ref. 1." It seems that even the authors of this paper were not convinced in this case that they obtained a pseudocapacitive electrode. Indeed, throughout Conway's paper the units used most often are C or μC cm−2 since "While it is of interest that the Co oxide film in its developed, reversibly chargeable, state behaves analogously to RuO2 or IrO2, and is multiply recyclable without degradation, it has the disadvantages, like IrO2, of (a) exhibiting a quite non-constant capacitance over its operable potential range and (b) having only a short (0.7 V) range for reversible charge admission and withdrawal." Fig. 3 depicts the CV of a Co3O4 thin film electrode in 1M KOH electrolyte. Although a pseudocapacitive behavior can clearly be observed up to 0.3 V vs Hg/HgO, above this upper potential limit a pair of well-defined redox peaks can be observed (Fig. 3), which are typical of the faradaic behavior of cobalt oxide in such electrolyte. Again, it does not make sense to calculate an "average" capacitance that will obviously not be constant throughout the whole potential window, but rather one should calculate the capacity in C g−1 for the electrode over the desired operating regime, which obviously depends on the targeted application. Such calculations will be essential in order to build a full device using a capacitive negative electrode, knowing that typically the capacity of the positive faradaic electrode must be oversized compared to the capacity of the negative one in order to improve cycling ability.
As for Ni(OH)2, there are hundreds of papers dealing with the pseudocapacitive nature of cobalt-based oxides or hydroxides, or even nickel-cobalt oxides or hydroxides, which clearly exhibit a non-linear dependence of the charge stored vs the potential window. Again, it is difficult to define a capacitance because such a metric will not be constant as expected for a true pseudocapacitive material. Not all the papers follow this mistaken nomenclature. There are some studies that clearly point out the faradaic nature of nickel-cobalt oxide electrodes, which are denominated as high-rate capability positive electrodes for asymmetric devices.45 Indeed such high-rate capability electrodes are of great interest when coupled with a capacitive carbon negative electrode to design asymmetric (hybrid) devices with improved rate capability.
Conclusions
Faradaic electrodes definitely exhibit electrochemical behavior distinct from that of pseudocapacitive electrodes. We propose that the term "pseudocapacitive" must be only used to describe electrode materials such as MnO2 that display an electrochemical behavior typical of that observed for a capacitive carbon electrode in mild aqueous electrolyte. It is confusing to use the same term for materials such as Ni(OH)2 or cobalt oxides that might exhibit high-rate capability, but with the electrochemical signature of a "battery" electrode. Obviously, the behaviors of many EC-relevant materials are more complex than can be completely described by a single term. Some compounds exhibit both mechanisms with a pseudocapacitive contribution coming from the surface properties and faradaic contribution coming from intercalation mechanisms for example, as can be found for birnessite-type MnO2.34,46 Such materials must be described in such a way that the readers can understand which mechanisms are involved. Additionally, in Conway's book there are also many references to pseudocapacitive materials. The examples provided include hydrous oxides such as RuO2, but also underpotential-deposition reactions, intercalation in compounds such as TiS2, and to conversion of an oxidized species to a reduced species in a redox system in solution. These three last examples obviously do not match the definition given later on in the book: "pseudocapacitance arises when the extent of reaction, Q, is some continuous function of potential, V, so that the derivative, dQ/dV, arises that has the properties of a capacitance".
We hope our explanations will shed some light to this topic and help authors to correctly address the description of their electrodes.
Acknowledgments
The authors acknowledge Dr. Annaïg Le Comte, Dr. Christophe Aucher and Alban Morel for providing figures and comments from their PhD manuscripts. J. W. Long acknowledges the U.S. Office of Naval Research.