Article Text
Abstract
Introduction Procedural pain in neonates is associated with impaired neurodevelopment. Whether hearing development is impaired, however, remains unknown. This study examined potential cause-and-effect relationship between neonatal pain and subsequent hearing loss in mice.
Methods Male C57BL/6J mouse pups received an intra-plantar injection of complete Freund’s adjuvant on postnatal day 7 or repetitive needle prick stimuli from postnatal days 0–7. Mechanical and thermal pain thresholds were tested between postnatal days 14 and 49. The auditory brainstem response test was used to determine hearing thresholds. The inner ear structures and dendritic morphology in auditory cortex were assessed using immunofluorescence and Golgi-staining. The effects of oxycodone, tropomyosin receptor kinase B agonists and antagonists were tested.
Results Neonatal pain resulted in impaired hearing in adulthood of both pain models No damage or synapse loss was found in the cochlea but increased dendritic spine density and reduced brain-derived neurotrophic factor level were found in auditory cortex in neonatal pain group. Oxycodone attenuated hearing loss and the associated changes in dendritic spine density and brain-derived neurotrophic factor changes in auditory cortex. A tropomyosin receptor kinase B agonist reversed neonatal pain-induced hearing impairment and decreased caspase 3 expression in auditory cortex. Administration of tropomyosin receptor kinase B antagonist in naïve mouse pups impaired hearing development suppressed phosphorylated-AKT, and increased caspase 3 expression.
Conclusion Chronic pain during the neonatal period resulted in impaired hearing in adulthood in mice, possibly via the brain-derived neurotrophic factor signaling pathway and dendritic spine pruning deficiency in auditory cortex.
- analgesia
- Pain Management
- Analgesics, Opioid
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Repeated procedural pain in neonates has been associated with abnormal neurodevelopment.
WHAT THIS STUDY ADDS
This study establishes a cause-and-effect relationship between chronic pain during the neonatal period and hearing loss.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Neonatal pain should be adequately controlled to minimize the long-term impact on hearing.
Introduction
Frequent neonatal procedural pain is associated with abnormal brain development including both motor and the cognitive systems.1 Observational clinical studies suggested that the neonatal pain result in long-term hearing loss. For example, the prevalence of hearing loss in preschool children who underwent heart surgery in infancy is 20-fold higher than in the general population.2 Hospital stay of more than 5 days in neonatal intensive care unit (NICU) has also been associated with an elevated risk of hearing loss in subsequent life.3–5 Thus, examining the relationship between neonatal pain and hearing loss is critical.
Neonatal procedural pain contributes to neuroplasticity in the developing brain, and alters the developmental trajectory of stress hormones.6 The neonatal period is critical for neuroplasticity and ultimately shapes the neural circuits in the peripheral and central auditory systems.7 During neonatal periods, the auditory system is vulnerable to abnormal experiences or factors, such as medical intervention and inflammatory stimuli. Alterations in neuronal morphology and branching patterns have been shown to underlie some developmental disorders involving the auditory cortex.8 For example, modifications in dendritic arborization and spines have been observed in pyramidal neurons of the auditory cortex in a rat model of noise-induced hearing loss.9 Interestingly, deafening-induced song degeneration in the adult male zebra finch is accompanied by rapid changes in spine morphology and synapse numbers in Area X, which is located in the basal ganglia and an important auditory process area.10 Thus, neuroplastic changes in the auditory system may underlie the link between pain stress and hearing development deficiency.
The brain-derived neurotrophic factor/tropomyosin receptor kinase B (BDNF/TrkB) signaling pathway is involved in inflammation, pain, and hearing development and plays an important role in the brain development and maintenance of neuronal plasticity.11 12 BDNF level rapidly increases by 10-fold in the central nervous system during the first postnatal 3 weeks. Within the auditory neuronal pathway, BDNF is first expressed in cochlear neurons, then in the brainstem auditory nuclei in the brainstem, and finally in the central auditory cortex.13 Based on this temporal pattern, this study aimed to identify the effect of neonatal pain on hearing development, its underlying mechanism, and its relationship with BDNF and associated molecules within the auditory neuronal pathway. We speculated that persistent neonatal pain could significantly influence the auditory neuronal pathway, ultimately resulting in long-lasting hearing impairment via the BDNF signaling pathway.
Methods
Animals
C57BL/6J mice (Nanjing Institute of Biomedicine, China) and knockout mice with no functional transient receptor potential cation channel subfamily V member 1 (TRPV1) (TRPV1-KO, C57BL/6JSmoc-Trpv1em1Smoc, Shanghai Model Organisms, Shanghai China) were housed in a standard small animal facility (22°C±2°C, 12–12 hour light/dark cycle) with free access to standard food chow and water.
Experimental design
This study consisted of five sets of experiments. In the first set of experiments, male C57BL/6J mouse pups (postnatal days 7, P7) received an intraplantar injection of 5 µL complete Freund’s adjuvant (CFA; Sigma-Aldrich, Germany) into the right hind paw. Thirty min before CFA injection, mouse pups randomly received an intraperitoneal (i.p.) injection of sufentanil (50 µg/kg, once per day), oxycodone (2 mg/kg, twice per day), or saline vehicle (twice per day) until P14 (figure 1A). The healthy controlled group of mouse pups received an intra-plantar injection of 5 µL saline. Thermal and mechanical pain thresholds were tested from P14 to P49. The auditory brainstem response (ABR) was tested on P28 and P56. The second set of experiments, male C57BL/6J mouse pups were randomly subjected to repeated needle pricks using a 23 G needle on all four paws (four times a day, separated by a 2-hour interval)14 or sham handling (with a cotton swab) from P0 to P7. Thermal and mechanical pain thresholds were tested between P14 and P49. ABR was tested on P28 and P56. In the third set of experiments, male C57BL/6J mouse pups administered with intraplantar injection of 5 µL CFA (P7) randomly received i.p. injection of the selective TrkB agonist 7,8-dihydroxyflavone (DHF) (5 mg/kg/day, Selleck, USA)15 or vehicle (3.3% DMSO) from P7 to P21. ABR was tested on P28. The experiments also included a group of mouse pups not receiving CFA at any time point, but receiving the selective TrkB inhibitor ANA-12 (0.5 mg/kg/day, i.p, Selleck, USA) or vehicle (3.3% DMSO in 1×PBS) from P7 to P21, but not receiving CFA on P7. The fourth set of experiments was conducted in TRPV1-KO mouse pups using a design similar to the first set of experiment. In the fifth set of experiments, intra-plantar injection of 5 µL CFA occurred on P28 instead of P7. Mechanical pain thresholds were tested on day 4, 7, 14, and 24 after CFA injection, ABR was tested on P56.
The CFA injection and repeated needle prick induced persistent pain during the auditory critical period and hearing loss in adult. (A) Schematic representation of the experimental design. (B) The comparison of the mechanical threshold between the Ctrl and CFA groups. (C) The comparison of paw thermal withdrawal latency between Ctrl and CFA groups. (D) CFA injection induced the ABR thresholds increment at P28. (E) CFA injection induced the ABR thresholds increment at P56. (F) Schematic and pattern representation of the experimental design of the needle prick model. (G) The comparison of the mechanical threshold between the Ctrl and NP groups. (H) The repeated needle pricks induced the ABR thresholds increment at P28. (I) The repeated needle pricks induced the ABR thresholds increment at P56. Panel (D, E, H, I), n=5–9, one-way ANOVA Dunnett’s post hoc test. *p<0.05, **p<0.01 and ***p<0.001 vs ctrl group. n=5–8, unpaired Mann-Whitney U tests were performed in B, C, and G. *p<0.05 and **p<0.01 vs ctrl group. ABR, auditory brainstem response; ANOVA, analysis of variance; Ctrl, control; CFA, complete freund adjuvant injection; PND, postnatal day; PWT, paw withdrawal threshold; PWTL, paw withdrawal thermal latency.
Auditory brainstem response
ABR was measured at 8000, 16,000, 24,000, and 32,000 Hz by an experienced observer who was blinded to the group allocation. Briefly, the mice were anesthetized with an intraperitoneal injection of ketamine (0.1 g/kg) and xylazine (20 mg/kg). ABR was recorded using a TDT-III system and analyzed using BioSig32 software (Tucker-Davis Technologies, USA). The hearing threshold tests was tested starting with 90 dB tone burst stimuli. The intensity of burst stimuli was reduced in a stepwise manner (5 dB for each step) until the lowest tone burst stimuli level was visually recognized and elicited an ABR.
Nociceptive behavioral tests
Thermal pain testing was conducted using a standard light heat device (IITC Life Science, USA) in the plantar region of the left hind paw (ipsilateral side of the CFA injection). Flinching, flicking, and trembling were considered as positive responses. Testing was conducted in triplicate at 5 min intervals, and the mean was calculated as the paw withdrawal thermal latency. Mechanical pain was tested using the up-and-down method with the von Frey filaments. Briefly, five consecutive touches were applied at 5 min intervals for rest. The filaments were pressed against the plantar surface and held for 3 s for a positive paw withdrawal response. Finally, the paw withdrawal threshold was calculated.
Immunofluorescence staining
Mice were deeply anesthetized and perfused with 4% paraformaldehyde. Brain sections (25 µm) were blocked with 3% donkey serum for 2 hours at room temperature prior to incubation with one of the following primary antibodies overnight at 4°C: BDNF rabbit antibody (1:200; Abcam, USA), glial fibrillar acidic protein (GFAP) mouse antibody (1:500; Cell Signaling Technology, USA), the neuronal nuclear protein (NeuN) mouse antibody (1:100; Cell Signaling Technology), and ionized calcium-binding adapter molecule-1 (IBA-1) goat antibody (1:500; Novusbio, USA). After washing with PBS, sections were incubated with an appropriate secondary antibody for 2 hours. The sections were mounted and observed under a Leica SP8 laser confocal microscope.
Cochlear whole-mount preparation
Mice were euthanized with an anesthetic overdose. After removing the temporal bones, round and oval windows were opened and perfused with 4% paraformaldehyde. After decalcification with 10% EDTA, the entire organ of corti was removed and dissected into upper, medial, and basal parts under a stereomicroscope. Whole-mount preparations were incubated with anti-myosin VIIa (1:400, polyclonal rabbit, Developmental Studies Hybridoma Bank, Iowa City, Iowa, USA) or anti-CtBP2 (1:200, monoclonal mouse, BD Biosciences, USA) overnight at 4°C before visualization with a 488-conjugated anti-rabbit secondary antibody (1:400, Cell Signaling Technology, UK) and an Alexa 555-conjugated anti-mouse secondary antibody (1:400, Invitrogen, USA).
Open field test
Open field test (OFT) tests were performed on P28 among CFA-induced neonatal pain mice, the control mice, and oxycodone plus neonatal pain mice, respectively. The test was conducted using a standard 40×40 cm arena made out of plastic. This field was artificially divided into a 20×20 cm center zone and a rest peripheral zone. The mice were not habituated to the arena before testing. The mice were placed in the center zone and allowed to travel freely for 10 min; the trajectory and speed of locomotion were recorded and analyzed using Smart V.3.0, a video tracking software system (Panlab, Span).
Golgi-staining and analysis
Unfixed mouse brain sections (100 µm) were prepared using a vibratome, mounted on gelatin-coated glass slides, stained using the FD Rapid GolgiStain kit (FD Neurotechnologies, USA), and examined under a transmission light microscope (Nikon Eclipse 80i, USA). Captured images were analyzed using ImageJ software and Sholl (NIH, https://imagej.nih.gov/ij/) to determine the dendritic length and density.
Statistical analysis
All statistical analyses were performed using GraphPad Prism version V.8.3.0 (GraphPad Software, California, USA). The distribution of continuous variables was examined using the Kruskal-Wallis test. Data are shown as mean± SE of the mean and analyzed using one-way analysis of variance, followed by Tukey’s or Dunnett’s post hoc test for multiple group comparisons. Statistical significance was set at p<0.05.
Results
Neonatal pain resulted in hearing loss in early adulthood
We established two different neonatal persistent pain models as previously described and then tested pain behavior and hearing threshold changes.14 16 CFA injection and behavioral testing are shown in figure 1A. CFA injection at P7 reduced the mechanical pain threshold significantly from P14 to P49 (figure 1B) and the thermal pain threshold from P14 to P28 (figure 1C). OFT test and contemporary body weight measurement results indicated that neonatal pain did not affect motor function or mouse growth development (online supplemental figure 1A,B). CFA resulted in significantly increased ABR threshold levels at 8000, 16,000, 24,000, and 32,000 Hz at P28 and P56, compared with the control group (figure 1C,D). CFA injection on P28, in contrast, did not affect hearing impairment (online supplemental figure 2).
Supplemental material
Repeated needle-prick stimuli were conducted from P0 to P7 (figure 1F), mimicking repetitive noxious stimulation among the infants administered in NICU. Needle-prick stimuli reduced mechanical withdrawal thresholds at P14 and P18 in neonatal mice (figure 1G). Compared with the control group, repeated needle-prick stimuli increased the average hearing threshold (at 16,000, 24,000, and 32,000 Hz) at P28, but not at P56 (figure 1H,I).
Analgesics prevented hearing impairment induced by neonatal painful stimuli
Next, we explore whether analgesics could rescue hearing loss by suppressing chronic pain. Administration of oxycodone within P7–P14 partially rescued the mechanical threshold decrease and ABR scores at P28 and P56 (figure 2). Also, the administration of another analgesic drug, sufentanil, marginally reversed hearing loss at P28 (online supplemental figure 3). Furthermore, In KO mice lacking functional TRPV1, a critical molecule in nociceptive transmission,17 the CFA administration also did not affect either pain threshold or hearing development (online supplemental figure 4).
The persistent neonatal pain-induced hearing loss was rescued mainly through the administration of oxycodone. (A) Schematic representation of the analgesic experimental design. (B) The administration of oxycodone rescued the mechanical withdrawal threshold decrease induced by CFA injection. (C) Oxycodone administration attenuated the ABR thresholds increase induced by the CFA injection at P28. (D) Oxycodone administration attenuated the ABR thresholds increase induced by the CFA injection at P56. (F–H) n=4–5, one-way ANOVA Dunnett’s post hoc test. *p<0.05, **p<0.01 and ***p<0.001 vs ctrl group; #p<0.05 and ##p<0.01 vs CFA+NS group. ABR, auditory brainstem response; ANOVA, analysis of variance; Ctrl, control; CFA, complete freund adjuvant injection; NS, normal saline; OXY, oxycodone; PND, postnatal day; PWT, paw withdrawal threshold.
Neonatal pain did not impair cochlear structural development
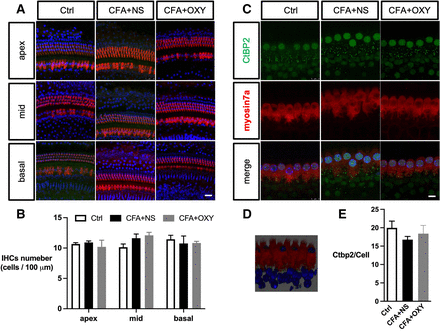
The impairment of the inner ear (cochlea) contributes to sensorineural hearing loss, thus, we evaluate whether the cochlear function was impaired by neonatal pain. Exposure to pain did not result in apparent morphological changes in any of the three subregions (apex, middle, and base) of the cochlea, as reflected by myosin 7a expression (figure 3A–C). The number of CtBP2 puncta was not affected (figure 3D–F). Moreover, administration of oxycodone within P7–P14 does not affect the cochlear morphology as well.
The CFA injection did not affect the cochlear hair cells and synapse structures. (A) Immunohistochemistry showed no significant hair cell loss apex, middle, or basal part of cochlear among groups. IHCs were labeled with myosin7a (red), and nuclei were stained with DAPI (blue). (B) The analysis of myosin-positive cells. N=3. (C) Cochlear synapse labeled with Ctbp2 of 3 groups. Scale bar=8 µm, n=3. (D) The 3D image showed the relative position of synapses and IHCs; synapses were located inside IHCs (E) The analysis of CtBP2 numbers per hair cell. Scale bar=25 µm. one-way ANOVA Tukey’s post hoc test. ANOVA, analysis of variance; Ctrl, control; CFA, complete freund adjuvant injection; IHC, inner hair cell; NS, normal saline; OXY, oxycodone.
Neonatal pain reduced BDNF level and increased spines density in auditory cortex
The auditory cortex is critical in processing auditory information, performing essential and higher functions in hearing. Besides, BDNF is widely expressed in the auditory system and affects spine pruning and maturation. We then detected BDNF expression in auditory cortex among the groups. BDNF colocalized with GFAP and NeuN, but not with IBA1 (figure 4A–D). Furthermore, both immunostaining and western blotting analysis revealed that pain significantly decreased BDNF expression in auditory cortex; this pain effect could be rescued by oxycodone (figure 4D, down, figure 4E).
The persistent neonatal pain induced by CFA injection decreased the level of BDNF expression in the auditory cortex. (A) Immunofluorescence double staining sho’ed AC’s colocalization of BDNF (red) and GFAP (green). (B) Immunofluorescence double staining showed the colocalization of BDNF (red) and NeuN (green) in AC. (C) Immunofluorescence double staining sho’ed AC’s colocalization of BDNF (red) and IBA1 (green). (D) The comparison of colocalization of BDNF with GFAP, NEUN, and IBA1 and the analysis (top) of BDNF expression among different groups (down). (A–D), n=4–6. (E) Western blotting and analysis data of BDNF level in AC at 4 w. n=4. F (2,9) = 3.877 (F) Golgi-stained layer II–III pyramidal neuron in AC and the analysis of dendritic spine density among different groups. n=4, one-way ANOVA Tukey’s post hoc test. F (2, 12) = 1.464. *p<0.05 vs ctrl group; #p<0.05 vs CFA+NS group. Scale bar=25 µm. ANOVA, analysis of variance; BDNF, brain-derived neurotrophic factor; Ctrl, control; CFA, complete freund adjuvant injection; NS, normal saline; OXY, oxycodone.
Next, we performed Golgi staining and calculated the dendritic spine density of the auditory cortex among groups. Compared with the control group, CFA injection increased dendritic spine density significantly in auditory cortex; the effects were attenuated by oxycodone (figure 4F).
Potential role of TrkB/AKT/caspase 3 signaling pathway involving in neonatal pain-induced hearing impairment
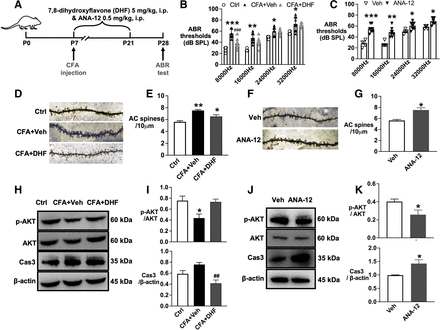
BDNF/TrkB-stimulated intracellular signaling is critical for neuronal survival and plasticity. For this, we tested the downstream signaling pathway of TrkB related to hearing loss. The ABR test data showed that administration of the TrkB agonist 7,8-DHF attenuated neonatal pain-induced hearing loss induced by CFA injection at P28 (figure 5A,B). Comparitively, the TrkB agonist ANA-12 in naïve mouse pups resulted in significant hearing impairment associated with the changes in dendritic spine density in the auditory cortex (figure 5C–G).
TrkB/ AKT/ Caspase3 signaling contributed to hearing loss induced by neonatal pain. (A) Schematic representation of drug administration and behavior design. (B) The administration of 7,8-DHF from P7~21 decreased the ABR thresholds in the CFA group at P28. (C) The administration of ANA-12 increased the ABR thresholds in naïve neonatal mice at P28. F (4,7) = 1.538 for 8000 Hz, F (4,7) = 3.482 for 16,000 Hz, and F (4,7) = 3.679 for 24,000 Hz. F (4,7) = 4.524, for 32,000 Hz. (D) Golgi-stained images showed that the administration of 7,8-DHF decreased the spines density induced by CFA injection. F (2,6) = 0.2365. (E) The analysis data showed the spines density difference between groups based on Golgi-stained images. (F) Golgi-stained images showed that the administration of ANA-12 decreased the spines density induced by CFA injection. F (2,2) = 4.722. (G) The analysis data showed the spines density difference between groups based on Golgi-stained images. (H) Western blotting images of p-AKT/ AKT/ Caspase 3. (I) The analysis data of western blotting images for p-AKT/ AKT/ Caspase 3. p-AKT/ AKT, F (2,9)=6.451. Caspase 3/actin, F (2,9) = 2.027. (J) Western blotting images showed p-AKT/AKT/caspase 3 changes induced by ANA-12 administration. p-AKT/AKT, F (3,3) = 3.237. Caspase 3/actin, F (2,2) = 1.46. (K) The analysis data of western blotting images. *p<0.05, **p<0.01 and ***p<0.01 compared with the ctrl or Veh group, #p<0.05, ##p<0.01 compared with the CFA+NS group. n=4–6, t-test was applied for panels F and K; the rest were applied for one-way ANOVA with Tukey’s post hoc test. AC, auditory cortex; ANOVA, analysis of variance; Ctrl, control; CFA, complete freund adjuvant injection; NS, normal saline; OXY, oxycodone.
TrkB-AKT signaling mediates the survival pathway that promotes CNS development. Western blotting analysis showed that 7,8-DHF administration decreased the protein level of caspase 3 in the auditory cortex in the CFA group. On the contrary, ANA-12 administration decreased the phosphorylated AKT and increased caspase 3 in the auditory cortex in naïve mouse pups (figure 4H–K).
Discussion
The evidence from this study established a cause-and-effect relationship between chronic pain during the neonatal period and hearing loss in early adulthood in mice, but the pain at a later development stage (CFA injection on P28) or in TRPV1-KO neonatal mice did not affect hearing. The hearing loss was associated with reduced BDNF levels and increased dendritic spine density in the auditory cortex. Treatment with analgesic agents or a TrkB agonist could rescue neonatal pain-induced hearing loss and reduce spine density in the auditory cortex. The possible role of the AKT/caspase 3 pathway may be in BDNF signaling and, ultimately, hearing is schematically shown in online supplemental figure 5. These results indicate the importance of pain assessment and management in infants in clinical settings.
The critical period for auditory development in mice is P14–P21. During this period, the plasticity of the auditory nerve system is susceptive to many factors. In this study, we used two non-similar neonatal pain models and confirmed chronic pain behavior and its effect on hearing development in mice; evidently, CFA mice experienced persistent pain during the critical auditory period and developed hearing loss in adulthood. A repetitive needle prick also resulted in hearing impairment. These findings were pain-specific since analgesics could attenuate the effects. Comparatively, CFA injection in adult or TRPV1-KO neonatal mice did not affect the hearing system. Thus, the behavior results indicated that neonatal pain during the critical period of auditory development is linked to hearing loss in adulthood.
The underlying mechanism of hearing loss may involve developmental plasticity impairment in the peripheral and central auditory pathways. The cochlea, sensitive to peripheral inflammation,18 plays an essential role in conveying frequency-specific acoustic information to the brain. Thus, inflammatory damage to the cochlea could be one of the potential contributors in hearing loss. However, in this study, we did not observe pathophysiological changes related to neonatal pain in the cochlea, indicating that the peripheral hearing system is not involved.
We then investigate the central auditory system mechanism focused on the typical sensory, along with auditory, experiences that drive spine pruning and stabilization during development.19–21 For example, dendritic pruning of striatal spiny neurons contributes to skill learning by increasing the signal-to-noise ratios. Our data showed that the increased number of spine synapses in the auditory cortex in mice subjected to neonatal pain might reflect the results of excess information pathways in local circuits on auditory deprivation. Thus, due to the immaturity of the descending modulation of nociceptive activity, preterm neonates may demonstrate dendritic pruning and central sensitization deficiency after repeated painful stimuli, which may, in turn, alter the brain microstructure and aberrant hearing development.
We found decreased BDNF levels in the auditory cortex in mice subjected to neonatal pain. BDNF promotes immature dendritic complexity and spine density in selected brain regions, contributing to sensory system develoment.12 13 For instance, BDNF participates in spine activity-dependent pruning and growth during normal cortical development in the visual cortex. Its overexpression in the visual cortex induces a critical period of sensitive plasticity.19 BDNF may trigger cortical inhibition in the auditory system and enhance spatial and temporal cortical resolution.
As BDNF is related to nociceptive stimulation, the expression of BDNF in different pathophysiological stages, brain regions, and cell types varies substantially. BDNF overexpression in the cochlea has a harmful effect during an injury but has a protective role in hearing development.22 First, BDNF gene expression is upregulated in the spinal dorsal horn and downregulated in the hippocampus during inflammatory pain.23 24 Second, instead of microglia, CFA-induced BDNF expression decreased mainly in neurons and astrocytes but not microglia in the auditory cortex regions in this study. Microglial-derived BDNF triggers harmful hyperexcitability of somatosensory nerves following peripheral nerve injury.25 One explanation is that the different steroid receptors that modulate BDNF gene expression in brain regions following stress-induced and early pain exposure could induce BDNF downregulation.26 27
Our study further explored TrkB/AKT/caspase 3 signaling in neonatal pain-related hearing loss. CFA injection induced downregulation of p-AKT and increased the caspase 3 in the auditory cortex, and TrkB agonist deteriorated these effects. AKT mediates neuronal survival by reducing caspase 3 activity, which is essential for regulating spine density and dendrite morphology.28 In a previous study in developing chick ciliary ganglions, caspase 3 activity significantly reduced the number of branching points, branching order, and complexity index.29 Thus, the inhibition of TrkB /AKT signaling and activation of caspase 3 may contribute to neonatal pain-induced spine pruning deficiency (online supplemental figure 5).
Our study has several limitations. First, we did not examine the potential sex differences, as suggested in previous studies.30 Thus, future studies should address this gender imbalance. Second, we did not investigate the role of auditory-related brain regions, such as the cochlear nucleus, the superior olivary complex, and the inferior colliculus, in neonatal pain-induced spine pruning deficiency.
Conclusion
Chronic pain during neonatal period could induce hearing impairment in early adulthood. Mechanisms underlying this phenomenon may include BDNF/TrkB signaling and dendritic pruning deficiency in auditory cortex. At a clinical level, neonatal pain should be adequately controlled.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
The Institutional Animal Care and Use Committee of Fudan University approved the experimental protocol.
Acknowledgments
We thank the Department of Anesthesiology, Renji Hospital, School of Medicine, Shanghai Jiaotong University, for supporting us with TRPV1-KO mice and for critical discussions. We thank the National Center for providing the use of Olympus spinSR, Pannoramic MIDI II, and Leica SP8, and we are grateful to Shan Sun, Fengming Liu, and Yao Li for their guidance.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
NL, BC and GJ contributed equally.
Correction notice This article has been corrected since it published Online First. The author affiliations and corresponding details have been updated. The open access licence has also been updated to CC BY.
Contributors NL conducted the experiments and manuscript writing, performed data analysis, figures preparation and interpretation. BC and GJ participate in experiments and performed data analysis, figures preparation and interpretation. RX, YX, CL and GL provide necessary technical support. WL and YH design and supervise the project, provided financial supports, writing and revision. WL and YH contributed equally. All authors read and approved the final manuscript. YH responsible for the overall content as guarantor.
Funding This study was supported in part by grants-in-aid for scientific research from the the National Natural Science Foundation of China (82271295), Natural Science Foundation of Shanghai (21ZR1411300) and Shenkang Clinical Study Foundation of Shanghai (SHDC2020CR4061) to Dr. Yuan Han; the National Natural Science Foundation of China (82171264), Shanghai Municipal Health and Family Planning Commission Research Project (2019SY015), Science and Technology Commission of Shanghai Municipality (21511102000), and the Medical Engineering Fund of Fudan University (yg2021-008) to Dr. Wenxian Li. The sponsors have no involvement in the study design, data collection, and interpretation, writing of the manuscript, or decision to submit the manuscript for publication.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.