Article Text
Abstract
Objective Mucosal-associated invariant T (MAIT) cells are the most abundant T cells in human liver. They respond to bacterial metabolites presented by major histocompatibility complex-like molecule MR1. MAIT cells exert regulatory and antimicrobial functions and are implicated in liver fibrogenesis. It is not well understood which liver cells function as antigen (Ag)-presenting cells for MAIT cells, and under which conditions stimulatory Ags reach the circulation.
Design We used different types of primary human liver cells in Ag-presentation assays to blood-derived and liver-derived MAIT cells. We assessed MAIT cell stimulatory potential of serum from healthy subjects and patients with portal hypertension undergoing transjugular intrahepatic portosystemic shunt stent, and patients with inflammatory bowel disease (IBD).
Results MAIT cells were dispersed throughout healthy human liver and all tested liver cell types stimulated MAIT cells, hepatocytes being most efficient. MAIT cell activation by liver cells occurred in response to bacterial lysate and pure Ag, and was prevented by non-activating MR1 ligands. Serum derived from peripheral and portal blood, and from patients with IBD stimulated MAIT cells in MR1-dependent manner.
Conclusion Our findings reveal previously unrecognised roles of liver cells in Ag metabolism and activation of MAIT cells, repression of which creates an opportunity to design antifibrotic therapies. The presence of MAIT cell stimulatory Ags in serum rationalises the observed activated MAIT cell phenotype in liver. Increased serum levels of gut-derived MAIT cell stimulatory ligands in patients with impaired intestinal barrier function indicate that intrahepatic Ag-presentation may represent an important step in the development of liver disease.
- antigen presentation
- hepatocyte
- liver immunology
- fibrosis
- t lymphocytes
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
What is already known on this subject?
Mucosal-associated invariant T (MAIT) cells are the most abundant T cell population in the human liver, exerting important regulatory and antimicrobial functions. They were recently shown to be implicated in liver fibrogenesis.
MAIT cells are activated by bacterial metabolites, presented on major histocompatibility complex-related molecule MR1.
Several liver diseases are associated with impaired intestinal barrier function and gut dysbiosis.
What are the new findings?
Primary human hepatocytes, hepatic stellate cells and liver endothelial cells can robustly activate blood-derived and liver-derived MAIT cells, and promote generation of active MAIT cell antigen (Ag) when provided with its bacteria-derived precursor.
Sera from healthy human volunteers contain ligands stimulating MAIT cells in MR1-dependent manner, rationalising the observed activated MAIT cell phenotype in human liver. Levels of stimulatory ligands are increased in portal and peripheral blood of patients suffering from portal hypertension in need of transjugular intrahepatic portosystemic shunt stent, and in blood of patients with inflammatory bowel disease.
Our results reveal previously unrecognised roles for liver cells from the sinusoidal environment in MAIT cell activation, and raise the possibility that intrahepatic Ag-presentation is an important step in tissue homeostasis and the development of liver disease.
How might it impact on clinical practice in the foreseeable future?
Given the limited therapeutic options for patients with progressive fibrosis, our finding that liver cell-mediated activation of MAIT cells is prevented by MR1 antagonists, offers a promising therapeutic approach. Modulation of the abundance of bacteria-derived MAIT cell stimulatory ligands present in the circulation might attenuate liver fibrosis as well.
Measurement of circulating MAIT cell Ag levels could be considered as part of a diagnostic test panel for assessing gut integrity in the context of inflammatory diseases.
Introduction
Mucosal-associated invariant T (MAIT) cells are the most abundant population of innate-like T cells in humans; they comprise up to 5% of T cells in peripheral blood and are found in high numbers in the liver and mucosal tissues.1–3 MAIT cells are restricted to the highly conserved major histocompatibility complex (MHC)-class I related molecule MR1.2 They express a semi-invariant T cell receptor (TCR) containing the Vα7.2 variable chain (Vα7.2-Jα33/12/20) paired with an oligoclonal TCRβ repertoire.2 4 MAIT cells recognise MR1-bound metabolites of the riboflavin (vitamin B2) biosynthesis pathway produced by many pathogenic and commensal bacteria.2 5 6 The most potent stimulatory MAIT cell antigen (Ag) is the pyrimidine 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil (5-OP-RU). It is formed by non-enzymatic condensation of the bacteria-derived precursor 5-amino-6-ribitylaminouracil (5-A-RU) with methylglyoxal derived from the glycolysis pathway.7 On TCR activation, MAIT cells secrete pro-inflammatory cytokines like interferon γ (IFN-γ) and tumour necrosis factor alpha (TNF-α) and acquire potent killing capacity; they consequently exert important immunoregulatory and antimicrobial functions.8 9 MAIT cells are further characterised by high cell-surface expression of CD161, interleukin-18 receptor (IL-18R) and IL-12R. Thus they can be activated by respective cytokines in TCR-independent manner.1 10–12 These features of MAIT cells provide protective innate immunity before an adaptive immune response has formed.
MAIT cells constitute up to 40% of human liver residing T cells,1 3 13 14 indicating their importance in liver physiology and pathogenesis of liver diseases. Liver MAIT cells secrete large amounts of IFN-γ, TNF-α and IL-17. Secretion of the latter follows repetitive IL-12 stimulation or occurs in response to IL-7.13–17 IL-17 acts as a fibrogenic cytokine that activates hepatic stellate cells (HSCs).18 The aforementioned cytokine expression profile suggests a detrimental role of MAIT cells in liver inflammation and fibrogenesis. This is supported by recent findings that lack of MAIT cells in MR1−/− mice protects against liver fibrosis, and that MAIT cells from patients with liver cirrhosis show alterations consistent with profibrotic activity.16 17
Apart from the locally produced endogenous cytokines, the liver receives cytokines and bacterial products originating from the gut and systemic circulation. Bacterial products may also enter the liver via the biliary tree. The responsiveness to bacterial products places MAIT cells at a central position in the immunological gut-liver axis, a notion supported by the finding that biliary epithelial cells (BECs) present Escherichia coli-derived Ag to MAIT cells.14 Recent studies, performed in mice, also argue for a potential of the MAIT cell Ag 5-OP-RU or its precursor to cross the intestinal barrier.19 20 Furthermore, plasma from patients with alcoholic liver disease (ALD), having increased gut leakiness, was found to activate MAIT cells more strongly than plasma from healthy subjects.21
It has been proposed that various hepatic parenchymal and non-parenchymal cell types act as liver-resident antigen presenting cells (APCs) for MHC class I/II and CD1d-dependent Ag-presentation.14 22 However, it has not yet been investigated whether cells from the sinusoidal environment, including hepatocytes, HSCs and liver sinusoidal endothelial cells (LSECs), that is, cells in direct contact with the blood entering through the portal vein, can present metabolite Ags to MAIT cells via MR1.
In the present study, we determine the localisation of MAIT cells within the human liver and characterise the potential of human liver primary cells to promote formation of active Ag from its bacterial precursor and to function as APCs stimulating MAIT cells. We examine the potential of serum derived from patients suffering from portal hypertension or inflammatory bowel disease (IBD) to stimulate MAIT cells in MR1-dependent manner. We test the ability of non-stimulatory ligands to block MAIT cell activation by liver APCs as a therapeutic intervention interfering with the profibrogenic function of MAIT cells.
Materials and methods
Resources used are listed in online supplemental tables 1-3. Further details of resources and methods not described in the main text (including preparation of bacterial products and synthetic MAIT cell Ag; quantitative real-time PCR; ELISA; flow cytometry analysis; mass spectrometry analysis; and D-lactate assay) are in online supplemental data file.
Supplemental material
Human samples
Written informed consent was obtained from each patient. The study protocol conforms to ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the local ethics committees. Information about patient characteristics of liver biopsy/resection samples used for analyses, patients with a history of transjugular intrahepatic portosystemic shunt stent (TIPSS) placement and patients with IBD, are in online supplemental tables 4-6.
Patient and public involvement statement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Preparation of primary cells from human samples
Primary liver cells (hepatocytes, HSCs, LSECs and BECs) were prepared from liver samples from patients undergoing liver resection at University Hospitals in Basel and Bern, Switzerland. Isolation and purification of cells followed established published procedures. Adipocytes and human umbilical vein endothelial cells (HUVECs) were extracted using published protocols. Identity of purified cells was verified by flow cytometry and bright field or immunofluorescence (IF) microscopy.
To enrich for blood-derived MAIT cells from healthy donors, either CD8+ cells were isolated using the Miltenyi positive selection kit, or negative selection was performed using Abs against CD45RA, CD62L, CD19, CD14, CD36 and TCRγδ.
For detailed protocols and references, see the online supplemental data file.
IF and immunohistochemistry staining of human liver sections and analysis of cell distribution
Cryopreserved human liver biopsy or resection samples from the biobank at the Department of Biomedicine (University of Basel, Switzerland) and the GI biobank (Translational Gastroenterology Unit and the Oxford University Hospitals Trust, Oxford, UK) were used. MAIT cells were either visualised by combined IF staining for CD3, Vα7.2 and IL-18R-α, or by chip cytometry including also additional markers (CD161, CD4, CD8). For visualisation of natural killer (NK) cells, defined as CD57+CD3− cells, paraffin embedded liver sections were used. For detailed staining protocols and antibodies used, see online supplemental data file and online supplemental table 1.
MAIT cell, non-MAIT T cell, NK cell detection and calculation of their distance to regions of interest (ROIs) within liver sample images was performed using QuPath. Using the ‘distance to annotation 2D’ spatial analysis tool, the distance of cells belonging to different cell classes to the ROI was calculated. Only liver biopsies with an intact structure and presenting enough ROIs were used for distance calculations.
Cell lines and MAIT cell clones
All cell lines and MAIT cell clones are listed in online supplemental table 2. This list also includes two liver-derived MAIT cell lines (MAIT-BEL-10, MAIT-BSL-19) generated in this work from fluorescence-activated cell sorting (FACS)-sorted MAIT cells obtained from normal liver tissue samples of two patients undergoing surgery for colorectal cancer metastasis.
Ag presentation assays
All assays with primary human cells used as APCs were performed in Roswell Park Memorial Institute (RPMI) medium containing 10% fetal calf serum (FCS; always the same lot number) to exclude non-specific effects of variable culture media. E. coli lysate or synthetic Ag was added to the APCs at indicated concentration for 2 hours prior to addition of MAIT cells at an APC to MAIT cell ratio of 1:4. Primary MAIT cells were used right after isolation, MAIT cell clone SMC3 and liver-derived MAIT cell lines at day 14 after restimulation. MAIT cell activation and cytokine secretion was evaluated after 16 hours of co-culture. Further details of APC assays with primary cells and established cell lines are described in online supplemental data file.
Measurement of antigenic potential of human serum
Serum from peripheral blood was collected from healthy donors, from patients with IBD and from patients with a history of or in need of TIPSS. Portal blood was drawn during the TIPSS procedure. For Ag presentation assays, 104 K562-MR1 cells were seeded in RPMI medium without FCS and 50 µl of human serum was added. In experiments testing inhibition by acetyl-6-FP, K562-MR1 cells were incubated with 5 µM acetyl-6-FP for 1 hour before serum addition. After 2 hours incubation with serum, 35 μg/ml anti-MR1 Ab was added when indicated, followed by 1 hour incubation before the addition of 5×104 SMC3 MAIT cells and further incubation for 16 hours. IFN-γ secretion was measured by ELISA. To calculate 5-OP-RU equivalents, control MAIT cell activation assays containing different concentrations of 5-OP-RU (0.006–25 pM) were included in each plate.
When measuring intracellular IFN-γ staining in MAIT SMC3 cells, experimental conditions were similar to those used for ELISA, except that final incubation after the SMC3 MAIT cell addition was for 7 hours in presence of 5 µg/mL Brefeldin A. In control experiments, 5-OP-RU (0.1–10 pM) was added instead of the human serum. IFN-γ positive MAIT cells were assessed by FACS.
Statistical analysis
Unless indicated otherwise, all graphs presented in the figures represent data from three or more independent experiments. Exact numbers of repetitions are indicated in figure legends. Unless indicated otherwise, values plotted in the column graphs are means+SD. Statistical analyses were performed in GraphPad Prism V.7 or R V.4.1.2. In order to use parametric tests, we log-transformed the response variables or applied square root transformation. We used t-test when comparing the means of two groups, otherwise we applied one-way analysis of variance (ANOVA) with Dunnet post-hoc test for cases where we compared multiple groups to single reference group, or we applied two-way ANOVA with Dunnet post-hoc test in order to take into account a paring variable. In figures depicting distances to ROIs, significance levels were obtained from one-way ANOVA with selected post-hoc comparisons (t-test), p values were adjusted with Benjamini-Hochberg correction for ‘multiple testing’.
Results
MAIT cells are dispersed within the parenchyma in healthy human liver
We investigated, by IF staining, localisation of MAIT cells using human liver samples without apparent histopathological abnormalities, originating from 15 donors (see online supplemental table 4). MAIT cells were identified as cells positive for CD3, TCR Vα7.2 and IL-18Rα, a robust combination of markers as IL-18Rα parallels high CD161 expression on MAIT cells (figure 1, online supplemental figure 1A–C).9 MAIT cells were distributed throughout the parenchymal space in the liver (figure 1A and online supplemental figures 1A,B). MAIT cells represented 5%–33% of liver T cells, considerably exceeding the frequency of Vα7.2-positive conventional T cells (figure 1B). MAIT cells were found in or in the immediate proximity of the sinusoids, and occasionally also within portal fields. To assess their zonal distribution, we performed digital analysis, measuring distances of MAIT cells and non-MAIT CD3+ cells to their closest portal fields and central veins. MAIT cells showed dispersed localisation, with >75% localising at least 200 µm away from either central veins or portal fields, confining most of them to the intermediary zone 2 of the hepatic lobule, with enrichment towards portal fields (figure 1C,D). In detail, 5% were in portal fields and 7% within 100 µm of portal fields. In contrast, non-MAIT T cells were more frequently (~30%) found within portal fields (figure 1D). We also examined distribution of NK cells, another immune cell population present in liver and found them to be dispersed, like MAIT cells, but without proneness towards portal fields (online supplemental figure 1D,E).
MAIT cells localise dispersedly to the parenchymal space in the healthy human liver. (A) Representative IF analysis of tissue section from a liver biopsy without histopathological abnormalities (patient C3). Colocalisation of CD3, TCR Vα7.2 and IL18-Rα (see higher magnification lower panels) identifies MAIT cells (yellow arrowheads). White arrow and blue arrowheads point at a portal field and central veins, respectively. Lower panels also show MAIT cells in proximity of TCR Vα7.2-negative and IL18-Rα-negative T cells (high magnification panels in colour and greyscale are shown). (B) Percentages of MAIT cells and non-MAIT Vα7.2+cells vs total CD3 +T cells in the healthy human liver (n=15), assessed as shown in (A). ****P<0.0001, Wilcoxon matched-pairs signed rank test. (C) Representative IF analysis (left panel), computational analysis (middle panel) and H&E staining (right panel) of a tissue section from a liver biopsy without histopathological abnormalities (patient C6). Yellow arrowheads in the left panel depict localisation of MAIT cells. The middle panel shows the same section with the regions of interest (ROIs) portal fields (depicted with brown circles) and central veins (depicted with green circles), as well as a colour heat map of cell distance (ie, any detected nucleus) to MAIT cells. (D) Upper panel: Violin plot of MAIT cell and non-MAIT CD3 +T cell distance to ROIs portal field and central vein. The box plots indicate the median and quartiles. Data were obtained from analysis of nine different liver tissues without histopathological abnormalities (five biopsies and four resection specimens). Significance levels obtained from one-way analysis of variance with selected post-hoc comparisons (t-test), p values were adjusted with Benjamini-Hochberg correction for multiple testing. The response variable ‘distance’ was transformed (square root) to keep also 0 distances in the analysis. Lower panel: Bar histogram pointing to percentage of MAIT cells localising at indicated distance to either portal fields or central veins. IF, immunofluorescence; IL 18R, interleukin-18 receptor; MAIT, mucosal-associated invariant T; TCR, T cell receptor.
Liver cell lines differ in their ability to activate MAIT cells
We analysed several parenchymal and non-parenchymal established cell lines for a potential to act as APCs for MAIT cells. Primary blood-derived CD8+ T cells served as MAIT cell source (gating on CD8+CD161++Vα7.2+ MAIT cells, see online supplemental figure 2A) and either riboflavin-synthesising E. coli or synthetic 5-OP-RU as Ags. In contrast to conventional APC line THP-1, we observed neither IFN-γ nor activation marker CD69 expression by MAIT cells co-cultured with hepatoma cells HepG2 and Huh7 exposed to a low concentration of E. coli (figure 2A), whereas higher concentration resulted in increased CD69 expression but no marked IFN-γ or TNF-α production (figure 2B and online supplemental figure 2B). MAIT cell activation by LSEC line TMNK-1 led to IFN-γ and TNF-α expression in response to E. coli while activation by HSC line TWNT-4 was weaker, detectable only at higher E. coli concentrations (figure 2C–D and online supplemental figure 2C). The effects of TMNK-1 and TWNT-4 cell lines were dependent on MR1 and partially on cytokines IL12/18 (figure 2E–F). Finally, we found that BEC H69 cells present Ag in an MR1-restricted manner while HSC line LX-2 had no activating effect (online supplemental figure 2D). All liver-derived cell lines, except LX-2, responded to pure stimulatory Ag 5-OP-RU (figure 2A,C and online supplemental figure 2E). We measured MR1 surface levels and MR1 messenger RNA in different cell lines. HepG2 and Huh7 hepatoma cells were very low in both assays (online supplemental figure 2F–G), consistent with their limited ability to activate MAIT cells.
Liver cell lines exert limited capacity to activate MAIT cells. (A–D) MAIT cell expression of IFN-γ (left panels) and CD69 (right panels) measured by flow cytometry. Blood-derived CD8 +T cells were co-cultured with different indicated cell lines in the presence of fixed Escherichia coli or 5-OP-RU. (A) THP-1, HepG2 or Huh7 cells exposed to fixed E. coli (1.5e7 CFU/mL) or 10 nM 5-OP-RU (n=4). (B) THP-1, HepG2 or Huh7 cells exposed to increasing concentrations of fixed E. coli (1.3e7–5e8 CFU/mL) (n=3). (C) THP-1, LSEC TMNK-1 or stellate TWNT-4 cells exposed to fixed E. coli (1.5e7 or 5e7 CFU/mL) or 10 nM 5-OP-RU (n=4). (D) TMNK-1 or TWNT-4 cells exposed to increasing concentrations of fixed E. coli (n=3). (E and F) LSEC TMNK-1 (E) and stellate TWNT-4 (F) cells in the presence of fixed E. coli (5e7 CFU/ml) exposed to different combinations of blocking Abs. MAIT cell expression of IFN-γ, TNF-α, and CD69 is shown in left, middle and right panels, respectively, (n≥5). Other details as in panels (A–D). CFU, colony forming unit; IFN, interferon; IL, interleukin; LSECs, liver sinusoidal endothelial cells; MAIT, mucosal-associated invariant T; 5-OP-RU, 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil.
All investigated primary liver cells act as APCs stimulating MAIT cells, with hepatocytes being most efficient
We next investigated the APC activity of major types of primary cells present in liver parenchyma. From surgically removed human liver specimens, we isolated primary hepatocytes, hepatic myofibroblasts/HSCs, LSECs and BECs and verified their identities by flow cytometry and/or microscopy (online supplemental figure 3A–C). Importantly, we observed surface MR1 expression in all investigated cell types (figure 3A and online supplemental figure 3D), though quantitative assessment was not possible due to high autofluorescence.
Primary human parenchymal and non-parenchymal liver cells express MR1 and present antigen to MAIT cells, with hepatocytes representing most potent APCs. (A) FACS histograms showing primary BECs, LSECs, HSCs and hepatocytes (HEPs) stained with anti-MR1 (red) or isotype-matched control antibody (filled grey). (B) IFN-γ production by MAIT cell clone SMC3 on co-culture with hepatocytes incubated with Escherichia coli lysate (3e7 or 3e8 CFU/mL) (n=4). (C) Representative FACS histograms, showing indicated markers on clone SMC3 in response to hepatocytes incubated with E. coli lysate (3e6–3e8 CFU/mL). Filled grey histograms correspond to negative controls (CTRL) lacking lysate. D–G) IFN-γ production by clone SMC3 in response to incubation with indicated liver cells and Ags. (D) Hepatocytes incubated with 5-OP-RU (0.01–10 nM). E. coli lysate (Lys; 3e8 CFU/mL) served as positive control (n=8). (E) HSCs incubated with E. coli lysate (6e7 or 3e8 CFU/mL) (n=5). (F) HSCs incubated with 5-OP-RU (0.01–1 nM) (n=10). (G) LSECs and BECs incubated with E. coli lysate (3e7–3e9 CFU/mL) (n=6). In panels B, D, E, F and G, the data (means±SD of measurements from three independent wells originating from the same patient) exemplify representative experiments out of 4–10 performed with cells obtained from at least three different donors. K562 cells overexpressing MR1 (K562-MR1) served as positive control APCs in panels (B and F). Anti-MR1 antibody (αMR1) was used in panels (B and D–F). (H) Two representative examples of synthetic Ag 5-OP-RU titration (0.01–3 nM) on liver APCs. Three independent measurements per dose are depicted. (I) Pooled results of all experiments performed as in panel (H), additionally including cells from adipose tissue (ADIPO, n=4; assembly of two experiments using adipose stromal cells and two experiments using differentiated adipocytes) and human umbilical vein endothelial cell (n=3). Shown are concentrations of 5-OP-RU needed to reach EC50 of IFN-γ secretion. Statistical significance of differences to HEPs was determined by two-way analysis of variance with Dunnet post-hoc test. (J and K) Representative examples of 5-OP-RU (0.01–10 nM) titration on liver APCs, in the absence or presence of Abs blocking either IL-12/23 (J) or IL-18 (K). One representative experiment out of two is shown in each panel. Other details are as in panel (H). IFN-γ production was always measured by ELISA. ADIPO, adipocytes; APCs, antigen presenting cells; BECs, biliary epithelial cells; CFU, colony forming unit; FACS, fluorescence-activated cell sorting; HSC, hepatic stellate cell; IL, interleukin; IFN, interferon; LSEC, liver sinusoidal endothelial cell; 5-OP-RU, 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil.
We first used MAIT cell clone SMC3, representing a pure homogenous MAIT cell population,23 to assess the capacity of primary liver cells to act as APCs. Hepatocytes, when incubated with E. coli lysate, activated the SMC3 clone in MR1-dependent manner as shown by IFN-γ secretion (figure 3B), upregulation of activation markers (figure 3C) and downregulation of TCR (online supplemental figure 4A). Some of the observed shifts were relatively small because of high baseline expression of activation markers on regular restimulations of clone SMC3 to induce its proliferation. K562-MR1 leukemia cells stably overexpressing MR1 served as positive control APCs (figure 3B and F). The robust MR1-restricted MAIT cell activation by hepatocytes was confirmed using pure synthetic Ag 5-OP-RU (figure 3D and online supplemental figure 4B). It was particularly interesting to test whether HSCs are able to activate MAIT cells since HSCs have been postulated as main drivers of fibrogenesis in the liver,18 and MAIT cells were also recently linked to liver fibrosis.16 17 HSCs treated with either E. coli lysate or 5-OP-RU activated MAIT cells in a MR1-dependent way (figure 3E–F and online supplemental figure 4C), arguing for a direct interaction between HSCs and MAIT cells as a factor contributing to the mechanism of their fibrogenic activity. Primary cell populations of BECs and LSECs were also able to present E. coli-derived and synthetic 5-OP-RU Ag to SMC3 cells in MR1-dependent manner (figure 3G–H and online supplemental figure 5A).
Use of a MAIT cell clone enabled us to quantitatively compare the potential of different primary cells to activate MAIT cells by determining Ag concentrations needed to reach EC50 of IFN-γ production. For both synthetic 5-OP-RU and bacterial lysate, we found hepatocytes to be the most efficient APCs, also when compared with adipocytes and HUVECs, included as examples of primary cells of non-liver origin (figure 3H–I and online supplemental figures 5B,C. The differences between various liver cells persisted when either IL-12 or IL-18 was blocked (figure 3J–K and online supplemental figures 5D,E). Inclusion of exogenous IL-12 or IL-18 increased MAIT cell activation, a known effect synergistic with TCR-dependent stimulation.10 24 The effect decreased on addition of anti-cytokine antibodies (online supplemental figure 5F,G). Since primary hepatocytes might secrete IL-7,13 15 leading to enhanced MAIT cell activation, we tested its effect using BECs and HSCs; IL-7 had no effect on MAIT cell clone SMC3 activity (online supplemental figure 5H).
Polyclonal blood-derived and liver-derived MAIT cells are also robustly activated by liver APCs
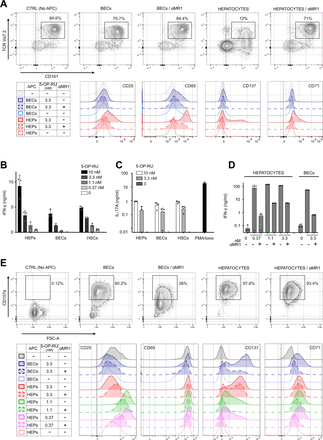
To investigate whether findings for the SMC3 clone apply to primary polyclonal MAIT cell populations, we isolated MAIT cells from human peripheral blood and liver tissue. Starting with peripheral blood mononuclear cells, which contain 2%–5% MAIT cells, negative magnetic bead selection depleted naïve T cells, B cells, monocytes, dendritic cells, platelets and γδ T cells, resulting in fourfold to sevenfold enrichment for MAIT cells (online supplemental figure 6A). Exposure of these enriched polyclonal MAIT cells to 5-OP-RU-loaded primary liver cells resulted in MR1-dependent downregulation of TCR expression, with hepatocytes again being very potent APCs (figure 4A, upper panels, and online supplemental figure 6B). Stimulation of blood MAIT cells by hepatocytes led to upregulation of activation markers CD25, CD69 and CD137, and the proliferation marker CD71 (figure 4A, lower panels). Consistent with the MAIT cell clone findings, BECs and HSCs were less potent APCs for blood-derived MAIT cells (figure 4A and online supplemental figure 6B).
Robust activation of polyclonal MAIT cells in response to interaction with primary human liver cell subsets. (A) Peripheral blood mononuclear cells, enriched for Vα7.2+CD161++cells by negative selection (see Materials and Methods, and online supplemental figure 6) were co-cultured with indicated liver APCs exposed to 3.3 nM 5-OP-RU. Upper panel: representative example of cell surface expression of CD161 and TCR Vα7.2 as measured by flow cytometry (n=3). Dot plots are gated on CD3 +CD8+CD26+cells. Lower panels: cell surface expression of CD25, CD69, CD137 and CD71, gated on CD3 +CD8+cells that are CD26 +and/or CD161+, as depicted in online supplemental figure 6C. (B and C) Production of IFN-γ (B) and IL-17 (C) by liver-derived MAIT cell line MAIT-BEL-10, measured by ELISA, in response to interaction with different liver APCs exposed to indicated concentrations of 5-OP-RU. Stimulation of MAIT cells with PMA/ionomycin (PMA/Iono) served as positive control. (D) IFN-γ production by liver-derived MAIT cell line MAIT-BSL-19, stimulated by indicated liver APCs exposed to 5-OP-RU (nM). (E) Cell surface staining for CD107a (upper panel), CD25, CD69, CD137 and CD71 (lower panels) on MAIT-BSL-19 cells, stimulated by either hepatocytes or BECs exposed to 3.3 nM (upper panel) or 0.37–3.3 nM 5-OP-RU (lower panels). Data in (D) and (E) are derived from the same experiment, which is representative of two experiments performed. MR1 dependence of activation was assessed with anti-MR1 blocking antibody (αMR1) in panels (A, D and E). Negative control (CTRL) in panels (A and E) lacks the APC. APC, antigen presenting cell; BEC, biliary epithelial cell; HEP, hepatocyte; HSC, hepatic stellate cell; IL, interleukin; IFN, interferon; MAIT, mucosal-associated invariant T; 5-OP-RU, 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil; PMA, phytohemagglutinin; TCR, T cell receptor.
To assess activation of polyclonal liver-derived MAIT cells, we generated two MAIT cell lines (MAIT-BEL-10 and MAIT-BSL-19) from two liver donors. Consistent with their tissue origin, the liver-derived lines expressed higher baseline levels of tissue residency marker CD69,13 compared with MAIT cell clone SMC3 (online supplemental figure 7A,B; see legend for additional characterisation of MAIT liver lines). In response to 5-OP-RU-treated hepatocytes, BECs or HSCs, the liver-derived lines produced IFN-γ and IL-17 and—as tested with hepatocytes and BECs—showed MR1-dependent upregulation of activation markers CD25 and CD137, and degranulation marker CD107a (figure 4B–E). Ag presentation capacity differed between different liver APCs (figure 4B and D–E). At high concentrations of 5-OP-RU, some of the hepatocytes’ effects were only partially blocked by MR1 antibody, possibly due to very abundant cell surface presence of MR1, caused by its stabilisation by 5-OP-RU,25 or a continual appearance of new MR1 on the hepatocyte surface (figure 4D–E). Moreover, we observed loss of CD25 high MAIT cells on stimulation by hepatocytes exposed to high (3.3 nM) 5-OP-RU, arguing for activation-mediated cell death (figure 4E, lower panel). High CD137 expression by MAIT cells stimulated by BECs confirmed their Ag-presentation capacity; however, induction of IFN-γ secretion was not as efficient as in response to hepatocytes (figure 4D–E).
To mimic an inflammatory context in which increased amounts of bacterial products and inflammatory cytokines reach the liver, synthetic Ag was mixed with E. coli lysate. Under these conditions, hepatocytes were again more efficient APCs than LSECs and BECs (online supplemental figure 7C), and this was paralleled by cytotoxicity elicited by the MAIT-BSL-19 cells (online supplemental figure 7D and online supplemental videos). Thus, as a proof of concept, we demonstrated that hepatocytes can be killed in vitro following Ag-mediated activation of liver-derived MAIT cells. Killing capacity by MAIT cells was also demonstrated for LSECs, using the SMC3 MAIT clone (online supplemental figure 7E).
Supplementary video
Supplementary video
Supplementary video
Supplementary video
Supplementary video
Supplementary video
Supplementary video
Inhibition of liver APC-mediated MAIT cell activation by MR1 blocking ligands
We explored whether blocking of MR1 occupancy with non-activating MR1 ligands prevents MAIT cell activation by liver cells, potentially offering an approach to counteract the postulated role of MAIT cells in liver fibrogenesis.16 17 We found that MAIT cell activation by Ag-exposed primary liver cells can be prevented in a dose-dependent manner by pre-treating liver cells with 6-formylpterin (6-FP) or acetyl-6-FP, known to enter the MR1 5-OP-RU binding pocket but not activate MAIT cells (figure 5A,B).5 26 The acetylsalicylic acid derivative 5-formyl-salicylic acid (5-F-SA) was found previously to stabilise MR1 on the cell surface, without stimulating Jurkat T cells overexpressing the MAIT TCR.27 We found that 5-F-SA decreased MAIT cell activation by liver APCs (figure 5B).
Non-activating MR1 ligands prevent MAIT cell activation by liver-derived APCs. MAIT cell clone SMC3 was co-cultured with indicated liver cells pretreated with different non-activating MR1 ligands for 1 hour before addition of 5-OP-RU. IFN-γ secretion was assessed by ELISA. (A) Hepatocytes either treated with 0.1–1 nM 5-OP-RU alone (CTRL) or pretreated with 50 µM 6-FP (left panel) or 5 µM acetyl-6-FP (right panel) before adding 5-OP-RU. (B) HSCs and BECs either treated with 5-OP-RU alone (CTRL), or pretreated with indicated concentrations of 6-FP, acetyl-6-FP, or 5-formyl-salicylic acid (5-F-SA) before 5-OP-RU addition. The mean+SD of measurements from three independent wells originating from the same patient’s cells is shown. One representative experiment out of two, originating from two different donors, is shown. APCs, antigen presenting cells; BEC, biliary epithelial cell; 6-FP, 6-formylpterin; 5-F-SA, 5-formyl-salicylic acid; HSC, hepatic stellate cell; IFN, interferon; MAIT, mucosal-associated invariant T; 5-OP-RU, 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil.
Primary liver cells promote active MAIT cell Ag formation when provided with its precursor
It has been established that the Ag precursor 5-A-RU is not able to bind to MR1 and activate MAIT cells.7 However, when 5-A-RU is used at high concentration, it can conjugate with cellular glycolysis products such as methylglyoxal, yielding active Ag.7 We observed that exposure of liver APCs to high concentration of 5-A-RU also Ieads to MAIT cell activation (figure 6A,B), suggesting that the formation of activatory 5-OP-RU is promoted by the liver APCs. Similarly, as in the case of the 5-OP-RU Ag presentation to MAIT cells (figure 3I), hepatocytes were more responsive to 5-A-RU than other liver and non-liver primary cells (figure 6B).
Liver-derived primary APCs promote generation of active Ag from precursor. (A) Representative experiment showing titration of the MAIT cell Ag precursor 5-A-RU (0.12–2000 nM) on different liver primary cells acting as APCs. IFN-γ production by MAIT cell clone SMC3 was assessed by ELISA. Three independent replicates per dose of 5-A-RU are depicted. (B) Pooled results of all experiments performed as shown in panel (A), using liver cells originating from at least three different donors per cell type, and also adipocytes (ADIPO) and HUVEC cells, analysed as shown in panel (A) (for more details, see legend to figure 3I). Shown are concentrations of 5-A-RU needed to reach EC50 of IFN-γ production. Statistical significance determined by two-way analysis of variance with Dunnet post-hoc test. (C) Quantification of 5-OP-RU by liquid chromatography tandem mass spectrometry in cell culture supernatants derived from indicated liver primary cells harvested after treatment with indicated doses of 5-A-RU (125–8000 nM). RPMI medium without inclusion of cells served as negative control (medium; assessed only at 8000 nM 5-A-RU concentration). Ag, antigen; APCs, antigen presenting cells; 5-A-RU, 5-amino-6-ribitylaminouracil; BEC, biliary epithelial cell; HEP, hepatocyte; HSC, hepatic stellate cell; HUVECs, human umbilical vein endothelial cells; IFN, interferon; LSEC, liver sinusoidal endothelial cell; MAIT, mucosal-associated invariant T; 5-OP-RU, 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil; RPMI, Roswell Park Memorial Institute.
We used liquid chromatography tandem mass spectrometry (LC-MS/MS) to follow formation of 5-OP-RU by liver cells. To standardise the system, we incubated THP-1 cells with 5-A-RU and detected time-dependent accumulation, in both cell lysate and supernatant, of 5-OP-RU (and its decay at later time points) by selected reaction monitoring (online supplemental figure 7A–D). LC-MS/MS analysis indicated that all tested primary liver cell types generated 5-OP-RU in a dose-dependent manner when provided with the precursor 5-A-RU (figure 6C).
The 5-A-RU conversion to active Ag in vivo in the liver depends on 5-A-RU crossing the intestinal barrier. To assess the probability of 5-A-RU crossing an intact barrier, we performed in silico modelling using a combination of physico-chemical parameters.28 29 Applying this method to the Ag 5-OP-RU and its precursor 5-A-RU, we found that of these two chemically very similar compounds the precursor has a higher probability of intestinal absorption than the active 5-OP-RU product (online supplemental figure 7E).
MAIT cell Ags are present in human serum and increased in patients with portal hypertension and IBD
To address the significance of our results in a physiological and pathophysiological setting, we performed measurements of MAIT cell stimulatory potential of human serum from healthy subjects, patients with portal hypertension undergoing TIPSS and patients with IBD (for patient details, see online supplemental tables 5 and 6). We measured IFN-γ secretion by MAIT cell clone SMC3, when stimulated by K562-MR1 cells exposed to serum. With sera from healthy volunteers (figure 7A), we observed MR1-dependent IFN-γ secretion. We next analysed sera from patients with a history of TIPSS placement (figure 7B), suffering from advanced liver disease with portal hypertension, a condition generally associated with impaired intestinal barrier function.30 31 For eight patients, serum was additionally available from both the peripheral blood (PER) just before TIPSS and the portal blood (PORTAL) collected during TIPSS procedure (figure 7C,D). All tested patient sera were found to stimulate MAIT cells in MR1-dependent manner. Peripheral sera of patients with a history of TIPSS (past TIPSS) showed higher stimulatory potential than peripheral sera from healthy donors (figure 7D, left panel). Peripheral and portal sera of three TIPSS patients led to a very potent IFN-γ production by MAIT cells and in four other patients the portal serum was up to threefold more active in IFN-γ induction than the peripheral counterpart (figure 7D, right panel). Peripheral and portal sera of TIPSS patients also harboured more stimulatory metabolites, when calculated as 5-OP-RU equivalents (figure 7E).
MAIT cell stimulatory ligands are present in serum of healthy human subjects and increase in patients with portal hypertension undergoing TIPSS and patients with IBD (A–C)stimulation of IFN-γ secretion by healthy donor and TIPSS patients sera is MR1 dependent. K562-MR1 cells, serving as APCs, were incubated with sera obtained from ten healthy volunteers (A), peripheral blood sera of nine patients with past history of TIPSS placement (B), and from peripheral (PER) and portal (PORTAL) blood of five TIPSS patients (C). Anti-MR1 antibody (αMR1) was used to assess MR1 dependency of MAIT cell activation. CTRL: controls in the absence of αMR1. Following incubation, APCs were co-cultured with MAIT SMC3 cells. Shown are IFN-γ measurements of five representative patients. (D) Summary of all results obtained from experiments as shown in panels (A–C), including additional sera from 12 healthy volunteers and seven TIPSS patients. IFN-γ values remaining after MR1 blockade were subtracted from the total measured IFN-γ produced for each sample. Values represent means of measurements with individual sera. Patients with a history of TIPSS (past TIPSS; n=16) are compared with 22 healthy donors (left panel). Significance was calculated using log transformed IFN-γ values and a student’s t-test. For eight TIPSS patients (T1 through T8), the PER and PORTAL blood values are shown (right panel). (E) Same as (D) but activity of different human sera is depicted as equivalents of 5-OP-RU. To calculate 5-OP-RU equivalents, MAIT cell activation assays containing different concentrations of 5-OP-RU were included on each plate used to test human sera. Significance was calculated using log transformed data and a student’s t-test. (F) Comparison of MR-1 dependent MAIT cell stimulatory potential of human sera from healthy donors and patients with IBD, with values corresponding to IFN-γ secretion (left panel) or 5-OP-RU equivalents (right panel). The patients with IBD are subdivided to two subgroups: 9 in remission (under treatment) and 15 with active IBD (under treatment). Values represent means of measurement with individual sera (two to six experiments per sample). (G) D-lactate levels in sera of healthy donors and patients with either history of TIPSS or active IBD. Values represent averages of multiple measurements for individual sera. In (F) and (G) patient samples were compared with the healthy control samples and significance was determined using log transformed data by one-way analysis of variance with Dunnet post-hoc test. APCs, antigen presenting cells; IBD, inflammatory bowel disease; IFN, interferon; MAIT, mucosal-associated invariant T; 5-OP-RU, 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil; TIPSS, transjugular intrahepatic portosystemic shunt stent.
To obtain additional evidence that patients with conditions associated with a leaky gut have higher levels of MAIT cell stimulatory Ags, we analysed sera derived from patients with IBD. The sera of patients with IBD with active disease induced significantly higher levels of MR1-dependent IFN-γ secretion, and harboured more 5-OP-RU equivalents, than healthy donors or patients with IBD in remission (figure 7F).
We also tested sera of the TIPSS and patients with IBD for the ability to induce IFN-γ expression as detected by intracellular staining. The FACS analysis revealed MR1-dependent accumulation of IFN-γ positive MAIT cells in response to patients’ sera and also 5-OP-RU used as control (online supplemental figure 9A–C). Notably, activity of sera originating from either control subjects or TIPSS and patients with IBD was inhibited by acetyl-6-FP, further strengthening the argument that activation is MR1-dependent and mediated by Ag, likely 5-OP-RU (online supplemental figure 9D). Additionally, we measured serum levels of D-lactate, a bacteria derived product,32 to assess gut leakiness in our TIPSS and patients with IBD (figure 7G and online supplemental figure 9E).
Taken together, our results show that ligands stimulating MAIT cells in MR1-dependent manner are present in the blood under healthy conditions and increase under the circumstance of elevated portal pressure and intestinal permeability.
Discussion
Here we present results of a comprehensive analysis of the activation of human liver-derived and blood-derived MAIT cells by different types of primary liver cells, in a process mediated by MR1-bound bacterial Ags. We demonstrate that all tested human primary liver cell types activate MAIT cells, with hepatocytes being most efficient; these cells also promote formation of active 5-OP-RU Ag, indicating that a process of both Ag accumulation and its presentation by APCs, can occur in the sinusoidal environment which is rich in the dispersedly localised MAIT cells. We further demonstrate the presence of MAIT cell stimulatory ligands in the circulation of healthy human subjects, and their increased levels in sera of patients suffering from portal hypertension and IBD. Increased levels of gut-derived MAIT cell stimulatory ligands in patients with conditions associated with a leaky gut barrier suggest that intrahepatic Ag-presentation could represent an important pathophysiological step in the development of liver disease. Demonstration that non-stimulatory MR1 ligands prevent excessive MAIT cell activation by liver APCs supports a concept of using them to prevent or treat liver fibrosis.
We found that purified primary liver cells (hepatocytes, BECs, LSECs, HSCs) can specifically activate MAIT cells in MR1-dependent manner (figures 3–4 and online supplemental figures 4–6). To date, only BECs have been shown to present Ag to MAIT cells.14 Hepatocytes were the strongest inducers of MAIT cell activation; the effect was potentiated by cytokines (figure 3J,K and online supplemental figure 5C,D). Importantly, our results on MAIT cell activation by HSCs add functional significance to the previous finding of MR1 expression on HSCs.16 In line with their ability to produce fibrogenic and pro-inflammatory cytokines, MAIT cells were recently shown to promote profibrogenic HSC activation.16 17 Our results suggest that the interaction between HSCs and MAIT cells reciprocally potentiates their profibrotic properties. Although we cannot exclude that cells contaminating our primary liver cell preparations contribute to the observed differences in APC activity, the procedures used for cell isolation and assessment argue against this possibility. Notably, all liver-derived cell lines, except one of the two tested HSC cell lines (LX-2), were able—to varying degree—to present Ag to MAIT cells (figure 2 and online supplemental figure 2).
Previous studies on MAIT cell localisation in human liver yielded divergent results, with MAIT cells being either found exclusively in the sinusoidal space, or predominantly within portal tracts.14 16 17 By thorough assessment by IF and digital analysis of healthy liver samples, we found some MAIT cells in portal fields, with most of them however localised dispersedly in the sinusoidal environment (figure 1 and online supplemental figure 1). Our results argue for an extensive interaction potential of MAIT cells with abundant liver cell types, both in sinusoidal and portal environments. In comparison to other T cells and also NK cells, the dispersed distribution with proneness towards portal fields represents a unique feature of MAIT cells. Further studies are required to assess potential differences in MAIT cell phenotype as a function of their localisation, local Ag presence and interactions with other liver cells.
We demonstrated that all investigated primary liver cell types can promote generation of active Ag 5-OP-RU when provided with 5-A-RU precursor (figure 6). As we found a signal corresponding to 5-A-RU when searching the published MS data set from blood of healthy mice,19 it is likely that metabolites of gut bacteria regularly reach the liver. Chemical properties of 5-A-RU and 5-OP-RU support their diffusion through the gut epithelium (online supplemental figure 8E), as—most importantly—do recent findings that gut delivered 5-OP-RU controls intrathymic development of MAIT cells in mice.20 This concept is reinforced by our findings of MAIT cell stimulation by sera from healthy individuals and patients suffering from leaky gut conditions such as portal hypertension and IBD (figure 7). Our findings corroborate and extend results of the study by Riva et al21 in patients with ALD, which showed increased plasma endotoxin levels, hyperactivated blood MAIT cells with defective antibacterial responses, and more effective MAIT cell stimulation by patients with ALD plasma as compared with healthy subjects, consistent with decreased intestinal integrity. However, the MR1 dependence of the MAIT cell stimulatory effects was not assessed by Riva et al,21 leaving it open as to what extent the reported response is due to MR1 ligands or to MAIT cell stimulatory cytokines. Our study demonstrates that stimulation of MAIT cells by sera from investigated cohorts is MR1 dependent and repressed by acetyl-6-FP, a non-stimulatory MR1 ligand. Although this data indicate that the stimulation is mediated by an Ag (presumably 5-OP-RU), additional studies are needed to determine the exact nature of MAIT cell activatory ligands present in the circulation.
The findings that bacterial metabolites produced in the gut reach—even in healthy individuals—the circulation and the liver, where they may be converted to active Ags, suggest that tissue resident MAIT cells are maintained in a continuous low-level activation state. This is supported by elevated expression of activation and exhaustion markers CD38, CD39, PD-1 and TIM3 on liver MAIT cells.13 16 17 Low-level MAIT cell activation and upregulation of tissue residency markers, such as CD69, may contribute to maintenance of MAIT cells in the liver. The proliferative capacity of MAIT cells is lower than of conventional T cells,1 33 which is also reflected by a dispersed rather than clustered localisation of MAIT in healthy liver (figure 1 and online supplemental figure 1). The high frequency and dispersed distribution of MAIT cells in an alerted, ‘ready-to-act’ state might protect the liver from incoming pathogens or increased amounts of microbial products, consistent with the documented antibacterial and immunomodulatory activity of MAIT cells.9 11 34 Constant low-level activation of MAIT cells and their close contact with hepatocytes could also have implications for tissue homeostasis in the liver. Recent studies performed with MAIT cells isolated from gut, lung or blood have shown that activation of MAIT cells via their TCR, as opposed to cytokines, is associated with induction of a tissue-repair gene expression programme.35–37 Consistently, MAIT cells were found to promote tissue repair either in vitro,35 or in vivo in a mouse model of skin injury.38
Contrary to ‘homeostatic’ stimulation via TCR ligands, full activation of MAIT cells requires additional cytokine-mediated signalling.35 36 This is likely to occur when higher amounts of microbial products enter the liver on disruption of the gut epithelial and/or endothelial barrier, as observed in liver diseases associated with gut dysbiosis,21 39–42 and this work. In response to increased gut-derived stimulatory signals, activated MAIT cells might enhance liver inflammation and contribute to immune-mediated liver pathologies, including liver fibrogenesis. Indeed, liver-derived MAIT cells produce large amounts of pro-inflammatory cytokines and the profibrogenic cytokine IL-17 when stimulated by liver-residing APCs (figure 4B–D). Secretion of IL-17 by MAIT cells increases in response to IL-7 produced by hepatocytes under inflammatory conditions.13 15 Our finding of hepatocytes acting as strong APCs suggests that, under inflammatory conditions, hepatocytes could activate and skew MAIT cells towards an IL-17 phenotype. Also, the tissue repair activity of MAIT cells could be dysregulated during liver inflammation and contribute to fibrosis development. An additional consequence of MAIT cell activation might be the killing of liver APCs, including hepatocytes (online supplemental figure 7 and online supplemental videos), thus potentially contributing to pathogenesis of liver diseases.
In view of the potential impact of MAIT cells in liver pathologies, we explored possibilities of preventing excessive MAIT cell activation by abundant liver-derived APCs. We found that MAIT cell activation by Ag-exposed primary liver cells can be alleviated by pretreating them with 6-FP, acetyl-6-FP or 5-F-SA (figure 5). Of note, acetyl-6-FP and 3-F-SA (a compound with similar characteristics to 5-F-SA), were shown to have an inhibitory effect on MAIT cells in a mouse lung infection model,27 and acetyl-6-FP was found to reverse the MAIT cell phenotype in mouse lung cancer,43 and improve metabolic parameters in obese mice.44 Our findings, together with a recent report that acetyl-6-FP accelerates regression of liver fibrosis in mice,45 support the concept of using inactive MR1 ligands in treatment of liver diseases. The effectiveness of hepatocytes and other liver cells in presenting Ag to MAIT cells makes this approach of particular importance, both for treatment and prevention of fibrosis.
The presence of MAIT cell stimulatory ligands in the circulation, both in healthy human subjects and, in increased amounts, in patients suffering from conditions with leaky gut barrier, suggests that intrahepatic Ag-presentation may represent an important step in the development of liver disease. Although differences seen between patient and healthy donor sera may not solely be due to increased levels of vitamin B2 biosynthesis intermediates, modulation of the influx of bacteria-derived ligands to the circulation, for example, by interfering with the vitamin B2 pathway, might represent an additional approach to attenuate liver fibrosis. Furthermore, measurement of circulating MAIT cell Ag levels could be considered as part of a diagnostic test panel for assessing gut integrity in the context of inflammatory diseases.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Ethikkommission Nordwestschweiz: Permits EKNZ 2016-01188 and EKBB Ref. 78/07; Yorkshire and Humberside, approval 16/YH/0247. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We are grateful to the patients who agreed to donate biological material for research purposes. We thank Professor G De Libero for sharing the MAIT cell clone SMC3 as well as the β2M-MR1 construct and Professor M Cella for sharing the MR1 Ab hybridoma (clone 26.5). We thank Professors S L Friedman, D Jefferson and N Kobayashi for providing us with cell lines, and Professor A Scherberich for supply of the adipocyte ASCs. We acknowledge the NIH Tetramer Core Facility, Emory University, Atlanta, Georgia, USA, for providing us with MR1 tetramer. The MR1 tetramer technology was developed jointly by Drs James McCluskey, Jamie Rossjohn and David Fairlie, and the material was produced by the NIH Tetramer Core Facility as permitted to be distributed by the University of Melbourne. We acknowledge the microscopy, FACS and histology core facilities at the Department of Biomedicine, University of Basel. We thank the medical doctors who helped in obtaining patient samples.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
MJL and HM contributed equally.
AK, TJ and MJ contributed equally.
Correction notice This article has been corrected since it published Online First. In the Materials and Methods section, “Measurement of antigenic potential of human serum”, the MR1 blocking antibody (anti-MR1 Ab) was used as the concentration of 35 ng/mL instead of 35 μg/ml (microgram/ml). This has now been corrected in both the online version and supplementary file. The open access licence has also been updated to CC BY.
Contributors MJL and HM: study design, experimental work, data acquisition and analysis, contribution to manuscript preparation. AK, TJ, MJ and KP performed experiments, contributed to acquiring and analysing data and writing of the manuscript. M-AM, IF, HM, KDM and UD: experimental collaborators. JV and GC: histological assessment of the liver samples. AH, MHH, EB, JHN, OK and CJZ: provision of samples. PK designed and supervised experiments and critically revised the manuscript. DS supervised experiments and critically revised the manuscript. MFS designed, performed, analysed and supervised experiments and wrote and revised the manuscript. MFS acts as guarantor for the present study. All authors reviewed and approved the final version of the manuscript.
Funding Financial support for this study has been granted by grants to MFS from the Swiss National Science Foundation (SNSF; grant numbers PZ00P3_167828 and PZ00P3_189490), and the following foundations (grant numbers not provided by foundation): Goldschmidt-Jacobson Stiftung, Julia & Gottfried Bangerter-Rhyner Stiftung, Swiss Life Jubiläumsstiftung, Olga Mayenfisch Stiftung, Freie Akademische Gesellschaft, Nora van Meeuwen-Häfliger Stiftung, Vontobel Stiftung, Uniscientia Stiftung; and by grants Wellcome Trust (109 965MA), Cancer Research Institute (grant number not provided by foundation), NIHR Biomedical Research Centre (grant number not provided by foundation), Oxford and NIHR Senior Fellowship (grant number not provided by foundation) to PK.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.