Article Text
Abstract
Introduction Digital health interventions (DHIs) have huge potential as support modalities to identify and manage cardiovascular disease (CVD) risk in resource-constrained settings, but studies assessing them show modest effects. This study aims to identify variation in outcomes and implementation of SMARTHealth India, a cluster randomised trial of an ASHA-managed digitally enabled primary healthcare (PHC) service strengthening strategy for CVD risk management, and to explain how and in what contexts the intervention was effective.
Methods We analysed trial outcome and implementation data for 18 PHC centres and collected qualitative data via focus groups with ASHAs (n=14) and interviews with ASHAs, PHC facility doctors and fieldteam mangers (n=12) Drawing on principles of realist evaluation and an explanatory mixed-methods design we developed mechanism-based explanations for observed outcomes.
Results There was substantial between-cluster variation in the primary outcome (overall: I2=62.4%, p<=0.001). The observed heterogeneity in trial outcomes was not attributable to any single factor. Key mechanisms for intervention effectiveness were community trust and acceptability of doctors’ and ASHAs’ new roles, and risk awareness. Enabling local contexts were seen to evolve over time and in response to the intervention. These included obtaining legitimacy for ASHAs’ new roles from trusted providers of curative care; ASHAs’ connections to community and to qualified providers; their responsiveness to community needs; and the accessibility, quality and appropriateness of care provided by higher level medical providers, including those outside of the implementing (public) subsystem.
Conclusion Local contextual factors were significant influences on the effectiveness of this DHI-enabled PHC service strategy intervention. Local adaptions need to be planned for, monitored and responded to over time. By identifying plausible explanations for variation in outcomes between clusters, we identify potential strategies to strengthen such interventions.
- health systems evaluation
- health services research
- cluster randomized trial
- other study design
- prevention strategies
Data availability statement
Data are available in a public, open access repository. Data are available on the Harvard Dataverse platform and can be accessed here: https://doi.org/10.7910/DVN/NSKFK2.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- health systems evaluation
- health services research
- cluster randomized trial
- other study design
- prevention strategies
Key questions
What is already known?
It is widely recognised that digital health interventions (DHIs) show overall modest and varied effects and that the effectiveness of DHIs in complex service settings is highly context-dependent.
However, there is poor understanding about how contextual factors work to influence outcomes.
Thus there is scant evidence available to policy makers and programme designers in resource-constrained settings about possible sources of variation in implementation and outcomes that may arise from their own DHI programmes in different local contexts.
What are the new findings?
Our paper identifies wide variation in implementation and effectiveness of SMARTHealth India, an AHSA-managed DHI implemented in 18 primary healthcare (PHC) clusters in rural India.
In our study setting, we identified key mechanisms of trust, acceptability and risk awareness and five mechanism-based explanations for how the intervention may have achieved its effects.
What do the new findings imply?
The strategies for strengthening PHC service strategy interventions that we identify will be of interest to those designing and implementing similar initiatives elsewhere, helping them think through the possible adaptations and outcomes of their own initiatives in their own local contexts.
For researchers, our findings underscore the importance of exploring and publishing heterogeneity of results, and of conducting flexible process evaluations, to help aid and enrich interpretation of overall trial results.
Background
Cardiovascular disease (CVD) is the leading cause of death in many low-income and middle-income countries (LMICs), including India.1 Despite established effectiveness and cost-effectiveness of known interventions to prevent and manage CVD risk, including screening, early diagnosis, blood pressure (BP) control medications, and lifestyle risk reduction, large evidence-practice gaps remain worldwide.2 Promising implementation strategies in contexts of low coverage of primary healthcare (PHC) include (1) ‘task shifting’, where community health workers are delegated some of the tasks traditionally performed by doctors (2) clinical decision support systems that enable health workers to more rapidly determine appropriate evidence-based treatment and (3) short messaging service text reminders.3 Digital health interventions (DHIs) have shown promise as suitable support modalities for these strategies, and projects using them have proliferated in recent years. However, findings from studies investigating effects of DHIs on outcome and process indicators for CVD risk reduction show overall modest and varied results.4 5
The UK Medical Research Council guidance on evaluation of complex interventions recommends shifting focus from identifying ‘what works’, to identifying how, for whom and in what circumstances evidence-based interventions are most likely to be effective.6 It also recommends process evaluations attending to implementation, causal mechanisms and contextual factors, and developing and refining hypotheses about how intervention-context interactions may produce variation in outcomes.6
In this study, we report findings from a process evaluation of SMARThealth India—a stepped-wedge cluster randomised controlled trial (cRCT) of a PHC service system strengthening strategy for CVD risk management in rural India. Stepped-wedge trials with embedded process evaluations are well suited to examining the influence of context on intervention effectiveness because of the potential to calculate effect measures in each cluster and examine consistency of effects.7 8 Consistency in effect across clusters may increase the strength of the overall finding, whereas inconsistency complicates interpretation.9 Such studies can contribute to the scant published data about contextual influences on effectiveness of PHC interventions for chronic disease in LMICs,10 and help build understanding about how contextual factors may influence intervention uptake and impact.11
The objective of our paper is to identify mechanism-based explanations for how and in what local contexts SMARTHealth India achieved its effects. Specific aims are to: (1) identify cluster-level variation in outcomes, (2) identify how and in what contexts the intervention was effective and (3) recommend potential strategies for strengthening PHC service strategy interventions in similar settings.
Methods
Setting and intervention
SMARTHealth India was a multifaceted intervention implemented in 18 government-run PHC facilities in West Godavari District in rural Andhra Pradesh, India. Each PHC facility services around 30 000 residents in surrounding villages, supported by PHC doctor, pharmacist, and nurse/mid-wife, and at the village-level, Accredited Social Activists (ASHAs) (1 per 1000 people). At trial commencement ASHAs were delivering predominantly maternal and child health outreach services under performance-based renumeration arrangements. The trial hypothesis was that a multi-faceted intervention involving capacity strengthening of PHC doctors and ASHAs through use of a mobile device-based clinical decision support system would result in improved BP control for people at high CVD risk when compared with usual care.12 The intervention strategy supported ASHAs to take new roles in identifying and following up people in their communities who were at high CVD risk and to facilitate referrals to government PHC facility doctors. A mixed-methods pilot study found the intervention was feasible and acceptable.13 Pre-trial modifications made in response to barriers identified in the pilot, included improved medication supply and support to PHC doctors to conduct dedicated village visits. Intervention details and the mHealth evidence reporting and assessment checklist have been previously published.12 14 15
cRCT design
The cRCT was implemented over 2 years duration (June 2014-June 2016). Eligibility, sampling and population characteristics have been previously described.12 13 In brief, clusters, defined at the level of the PHC facility, comprised the facility and all ASHAs in selected villages. To be eligible for inclusion, PHCs needed to be within 40 km from a major town. Clusters crossed over from control to the intervention arm in one of three steps at 6 monthly intervals. This resulted in intervention periods of 18, 12 and 6 months for PHC clusters allocated in steps 1, 2 and 3 respectively. Quantitative outcomes were assessed by independent data enumerators. At baseline, using the same criteria as used by ASHAs, enumerators identified a high-risk cohort from each village from a complete baseline household sample. They collected outcome data through repeat cross-sectional surveys of independent samples of 15% of this high-risk cohort—conducted at each step of the trial (average ~150 per cluster per step). All individuals identified as being at high risk were eligible for further assessment by the ASHAs and (where indicated) on-going follow-up. The primary outcome was the proportion of the independent sample achieving optimal BP control (systolic BP <140 mm Hg). The previously published main trial results found improvements in BP control in both the intervention and control trial periods, and an overall null intervention effect.14 The intervention was also associated with a small improvement in guideline-recommended prescribing of medicines.14
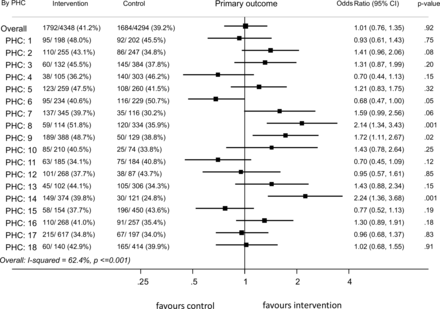
Forest plot showing OR for primary outcome in individual clusters in the intervention period compared with the control period. PHC, primary healthcare.
Process evaluation design
We used a mixed-methods explanatory study design, with a sequential component.16 Drawing on principles of realist evaluation,17 and the RE-AIM framework for evaluating public health interventions,18 we developed an initial theory of change to explain the influence of contextual factors on cluster level variation in outcomes (online supplemental additional file 1). These frameworks guided the first stage of qualitative data collection. Quantitative data were collected as part of the cRCT and outlined above.From the DHI, we extracted data on ASHAs’ screening and follow-up activities and numbers of PHC doctor visits. The first phase of qualitative data collection comprised 13 focus group discussions with ASHAs, and 12 in-depth interviews with ASHAs, PHC doctors and high-risk community members from 13/18 clusters. Interviews and focus groups were conducted in Telugu, recorded, transcribed verbatim and translated into English for analysis. These data contained extensive and comparable context-related information that enabled cross-site analysis. Following initial qualitative and quantitative analysis of these datasets, the lead author (GS) then interviewed field team managers and the lead trial researcher (DPr) to elicit their understanding of factors affecting variation and to test emerging themes. These interviews included in-depth discussion of three purposively selected PHC clusters—a ‘positive’ cluster, an ‘inverse’ cluster and ‘average/null’ cluster—selected on the basis of intervention effects, and taking into account distribution across block allocation (PHC 8, PHC 6 and PHC 12 in figure 2). We sought possible explanations for outcomes achieved in these clusters, comparing and contrasting context and implementation between them and with other clusters showing similar outcomes.
Supplemental material
Primary and secondary outcomes by cluster and block allocation. BP, blood pressure; PHC, primary healthcare.
Analysis
We brought qualitative and quantitative data together at cluster-level, using a framework matrix analysis approach.19 The quantitative cluster-level analysis followed the same approach as used for the overall outcome analysis.14 We presented measures of association as ORs with 95% CIs and used forest plots to visualise cluster-level variation. PHC facilities were grouped based on the point estimates of the intervention effects into ‘strong/moderate’ (OR >1.99); ‘weak’ (1.29> OR ≤1.99); ‘null’ (0.8> OR ≤1.29) and ‘inverse’ OR ≤0.8. The quantitative analysis was conducted separately by one of the authors (QL). Qualitative data analysis was conducted by two researchers (GS and BP) who had not been involved in the main trial. They familiarised themselves with transcripts and developed analytical memo and an initial coding framework using a combined inductive and deductive approach, modified in subsequent coding rounds. The first stage of coding was conducted blinded to the quantitative results. Framework matrix analysis was used in the second coding round and categories and emerging themes refined by comparing and contrasting within and across clusters according to the initial theory of change (online supplemental additional file 1).11 20 Differences of interpretation were resolved by triangulating findings with qualitative and quantitative data and by examining data disaggregated by village. The main coder (GS) used QSR NVivo V.11 software (QRS, Vic, Australia) to manage the data, and exported matrices into Excel to share with other study authors. Our understanding of the influence of contextual factors and their interactions in subsequent within-cluster and between-cluster analysis, was enriched through contextualising our findings in relevant literature and with reference to a previously developed mid-range theory (MRT) of free public healthcare seeking.21 We chose this MRT from the large number of healthcare access, behaviour change, and implementation frameworks and theories available because unlike most of these, it offers an LMIC-derived, integrative and dynamic rather than fragmented explanation of healthcare use. Briefly, this theory posits that users’ choice to seek free public healthcare is found at the intersection of three mechanisms: trust, risk awareness and acceptability. Individual, local and structural ‘conversion factors’ interact with these mechanisms (‘triggering’ or ‘inhibiting’ in realist terms), to produce health outcomes by expanding or contracting users’ capability space and thus use of free healthcare. We focused on the influence of local contextual factors (‘local conversion factors’ in this MRT). Our view of context, consistent with this MRT, is that it is dynamic and an integral part of an intervention, rather than a static backdrop to it.22 Based on our analysis, and repeated questioning of the data, we iteratively developed mechanism-based explanations, as refined programme theory, to explain how and why the intervention achieved its effects in different local contexts.
Patient and public involvement
Patients and healthcare providers were involved in the intervention design through a feasibility phase. The outcome measures that were selected for the trial are real-world measures that reflect established pathways to adverse health events and premature mortality, and thus highly relevant to patients’ priorities. Qualitative interviews in the process evaluation included open-ended questions to elicit implementer and patient experiences with the intervention. Once the process evaluation findings are published, a plain language summary will be developed and provided to the district health department.
Results
Description of site-level variation in trial outcomes
There was substantial between-cluster variation in the primary outcome (figure 1). Nine clusters were assessed to have a ‘moderate’ (OR >1.99) or ‘weak’ (OR >1.29) intervention effect on the primary outcome. Of these, eight also showed a positive intervention effect on one or more of the secondary outcomes. Six clusters each showed positive intervention effects for both BP control and use of BP lowering medication, and for both BP control and increased vigorous physical activity (figure 2). PHC clusters showing positive intervention effects included clusters from all three trial steps (figure 2). Most clusters (14/18) had a marked increase in the proportion of people achieving BP control during step 2 of the trial. This coincided with a severe heatwave in the regions May–June 2015, discussed in the main results paper. Of these clusters, 10/14 sustained improvements during subsequent intervention periods, and 4 reverted to BP control levels similar to their baseline.
ASHAs’ delivery of screening services was consistently high with 84% population coverage or higher in all PHCs and they followed up 64%–99% (overall 85%) of those identified as at high CVD risk at least once (table 1). Follow-up by government PHC doctors ranged from 48% to 89% (overall 69%). There was a clear pattern of lower absolute and less frequent follow-up by both ASHAs and government PHC doctors in block 3 PHCs compared with blocks 1 and 2 (table 1). From our qualitative data, those at high CVD risk used a range of local health providers for follow-up, in addition to, or instead of the government PHC provider, and accessibility and capacity of providers to manage CVD risk was not static during implementation. In brief, and referred to at various points below, other health services consulted by those assessed to be at high CVD risk were ‘service 104’—a government-run private and public partnership providing a mobile health outreach service and provision of free essential medicines; private and government-run hospitals; general practitioners and specialists in private practice; and rural medical practitioners (RMP), informal providers unqualified to prescribe or manage BP control medications.
Characteristics of PHCs and intensity of programme implementation
Overview of understanding variation
The observed heterogeneity in intervention effectiveness was not attributable to any single factor, but was the result of different domains of influence, whose interactions and relative strength exerted both ‘positive’ and ‘negative’ influences. We identified five mechanism-based explanations for how local context interactions with SMARTHealth India produced outcomes—presented below and shown in figures 3 and 4.
(A) CMO configuration: obtaining legitimacy from higher level providers and the DHI—‘the public believes’. (B) CMO configuration: Responsiveness to community needs—‘we cannot go so far so you only get the medicines for us’. ASHAs, Accredited Social Activists; CMO, context, mechanism and outcome; DHI, digital health interventions; PHC, primary healthcare.
(A) CMO configuration: Developing risk awareness—‘because you warned me, I went’. (B) CMO configuration: Working with provider choice—‘you tell us to go there but they are not giving’. (C) CMO configuration: Influencing community members’ attitudes to medicines—‘why is it not showing an effect on me?’. ASHAs, Accredited Social Activists; CMO, context, mechanism and outcome; CVD, cardiovascular disease; PHC, primary healthcare.
Key outcome: community take up ASHAs’ new roles in screening and follow-up for CVD risk
Obtaining legitimacy from higher level providers and the DHI: ‘the public believes’
This theory posits that in the context of support by government PHC providers for ASHAs’ new roles (C1), trust and acceptability (M) are important for community uptake of the new roles—that is, agreeing to be screened and followed up (O), but where such support is lacking, legitimacy from other providers (C2), or appeal to technology (C3), enables trust and acceptability (M).
In PHC 9, where there appeared to be a good relationship between ASHAs and the government PHC facility doctor, and predominant service use was either the government facility, or private providers, the PHC doctor and ASHAs developed a shared understanding about working together in CVD risk management. ASHAs described assembling high risk people in a particular place and codelivering services with the doctor in an outreach setting: ‘we would conduct the camp in one place each time and call the doctor and tell everyone has come, then the doctor would come and check’ (ASHA FGD PHC 9). These ASHAs described their role as being in community, and having sufficient time to educate about CVD risk ‘The doctor would not speak to a patient for half an hour, right? But we would be able to go the neighbours and tell all those things in detail right?’ and were assured that the doctor would demonstrate his support when needed: ‘the doctor would say whenever you tell me, I'll come’ (ASHA FGD PHC 9). While codelivery had a practical element to it—people identified at high CVD risk could be immediately attended to—it also, we hypothesise—had the effect of building legitimacy for ASHAs’ new roles, with codelivery being a practical outworking of the social process of cooperation in which actors share a common goal and the means to achieve it.
In PHC 16, with lesser engagement of the government PHC facility doctor, and initial resistance from the community who did not want screening without treatment, arguing that ‘now you are seeing, but what are you giving us,’ (ASHA FGD PHC 16) ASHAs aligned themselves with service 104 rather than with the PHC facility, and this alignment provided legitimacy for their roles. They described how community consultation with service 104, and the provision of medicine by service 104, helped to overcome initial distrust in their new roles: ‘later on they spoke to the 104 people and when there sir, after coming there and taking the medicines from 104 they gained the complete trust in us sir’ (ASHA FGD PHC 16). In some local areas, service 104 and the PHC work more closely, for example, sharing medicines, but this was not the case in this locality. Here, alignment with service 104 was particularly useful to the ASHAs—service 104 being popular with community members on account of its provision of monthly supplies of free medications and proximity to the villages that it visited. Despite that more than three quarters (76%) of those identified by ASHAs as being at high CVD risk attended the government PHC facility for CVD risk management at least once (table 1), the doctor interviewed from this facility expressed the view that ASHAs were not suited to the task of identifying people at high CVD risk. In contrast to their perception about the value of taking time to educate community members, he asserted that ASHAs should be replaced with graduates so that ‘we can do the work easily and fast’ (MO IDI PHC 16). Thus, in this PHC, both service 104 and the PHC facility were engaged in the intervention, with service 104 providing the ASHAs with legitimacy, and provision of medication for some of those at high CVD risk, with the PHC doctor also engaged in follow-up care. In PHC 2, where neither the PHC facility nor service 104 provided overt support to them, ASHAs deliberately cited personal access to a remote doctor, apparently as a way to gain trust: ‘[we say] we feed in the tab and it goes to Bhimavaram, there the doctor examines and sends us back the report… the public believes and says Ok, amma, please do’ (ASHA FGD PHC2). Screening coverage was low here relative to the other PHCs (though still high at 85%).
Responsiveness to community needs: ‘we cannot go so far so you only get the medicines for us’
This theory posits that in the context of experienced ASHAs with strong community connections (C1), trust in ASHAs’ new roles (M) is further enabled by ASHAs extending their new roles beyond protocol in response to community needs (C2). This may create a ‘virtuous cycle’ where favourable context is reinforced by trust, uptake of the new service, resulting in stronger community connections (O).
ASHAs’ prior experience appeared to influence their responsiveness to community needs, and thus acceptability. As described by an experienced ASHA: ‘since we are in this program… if there is the mother-in-law, we say, ‘dear sister you are happy, going to have a grandson…if we speak like this, they used to ask us to come and check.’ (ASHA FGD PHC7). For some experienced ASHAs, project work not only built on their pre-existing relationships, but also strengthened and extended these relationships: ‘they have become close to us by this program… they even come to us for advice’ (ASHA FGD PHC14). In five clusters ASHAs took on an additional task of collecting BP control medicines on behalf of some community members. In reflecting on why ASHAs had done this in some clusters, but not others, the field team manager believed that this reflected particularly high levels of personal commitment to the intervention by these ASHAs. In PHC 8, he also explained that a precedent may have been set from a previous health programme. ASHAs from this cluster explained medicine collection in terms of needing to complete the loop of recommended treatment, and in terms of responding to community requests: ‘you are prescribing the medicines, but many of the aged people are not able to bring the tablets, that’s how they express their grief… They used to tell us that we cannot go so far so you only get the medicines for us…as such we used to get them sometimes’ (ASHA FGD PHC8). Some ASHAs screened more people more often than specified, in response to community requests. Requests for additional screenings arose during household visits ‘now if we check the wife and the husband’s name is not there, they would ask to check them also’ (ASHA FGD PHC3).
While delivering services household to household is a key feature of how ASHAs work, some ASHAs described delivering certain aspects of the intervention from their own homes or other locations, as described by ASHAs from PHC 6: ‘if we sit at home 5–6 people will come with Aadhar (government identity) cards…people take leave from their work and come visit us to get their BP, sugar checked, and get medicines.’ (ASHA FGD PHC 6). In this cluster, there had been few government experienced ASHAs available to work on the project, and women were recruited from the community to fulfil the ASHA role, using the same criteria as used to recruit to the ASHA programme. From our qualitative data, these less experienced ASHAs appeared to work differently from those who were already experienced in the Government programme, and from table 1, this cluster with a notable higher proportion of less experienced community recruited women in the ASHA position also followed up a lower proportion of those at high CVD risk than other PHCs having similar implementation periods.
In addition to ASHAs effectively delivering their project roles, effectiveness in terms of trial outcomes, required that the high-risk cohort accessed and adhered to appropriate quality care from higher level providers—discussed below.
Key outcome: high-risk cohort uses and adheres to recommended care
Developing risk awareness: ‘because you warned me, I went’
This theory posits that where ASHAs have good local knowledge of their communities and of local health services (C1), they draw on this knowledge to tailor their communication to community members assessed to be at high CVD risk, developing risk awareness (M), and influencing their decision to seek higher level medical care (O). Further, where ASHAs were seen to be involved in acute care (eg, support to a person with an acute CVD event to navigate emergency care) (C2), this strengthened risk awareness (M), influencing decisions of others in the community to follow recommended actions (O).
In PHC 9 where ASHAs had the support of the PHC facility doctor (described earlier), and where those at high CVD risk were seen to use a range of healthcare providers, including a large NGO and ‘service 104’, an ASHA described making numerous attempts to convince a stonemason to attend follow-up care. After learning from him that the man’s reluctance to attend for care stemmed from a fear of losing income, the ASHA tailored her communication to his situation, linking the importance of a doctor’s visit to the man’s expressed desire to provide for his family, thus successfully convincing him to attend: ‘I warned him saying you will be able to earn only when your limbs are healthy…once you get sick and get bed ridden, your whole family will suffer so go to the hospital. Then the man went to the hospital and got the medicines and then …[he] said, ‘because you warned me, I went and got the medicines’ (ASHA FGD PHC 9). In another instance, noting that some people did not use the PHC facility for anything ‘serious’, these ASHAs referred individuals with this care seeking pattern to private providers, giving a clear message of the seriousness of high CVD risk: ‘…once we told them to go to the private doctor and get the tests done, when the disease was shown to be real, then they would say we had never taken a tablet nor knew that any disease existed until you checked us’ (ASHA FGD PHC9). In addition to tailored communication, ASHAs in PHC9 described their concerted efforts to get people to attend for higher level medical care. From their descriptions of activities, ASHAs in different clusters may have differed in the emphasis placed on the importance of medical follow-up relative to self-management, with those better connected to local facilities, more likely to emphasise the importance of medical follow-up. In PHC 14, where ASHAs appeared to have good linkages with a range of local health providers, including a local RMP who got himself checked by them, the referral cards were appreciated—‘we liked the follow up sir. when we write the card, they get themselves checked based on the card’ (ASHA FGD PHC 14). In contrast, ASHAs from PHC 6, a cluster with a higher than expected proportion of community recruited women working on the project, and limited relationship with the PHC facility, when asked about use of higher-level medical care described the communication as follows:
‘A: - Everyone says the same what difference does it make if we go?
I: What would you tell them? tell them it’s compulsory?
A: we advise them to reduce salt consumption and chilli powder consumption take more green leafy vegetables’ (ASHA FGD PHC 6).
In some clusters, ASHAs assisted individuals at extreme CVD risk (for example extreme elevation of BP) to access emergency care. From our data, these instances were pivotal in developing heightened awareness of the seriousness of CVD events, and perceptions of personal susceptibility. From the ASHAs’ rich descriptions, it was not just the occurrence of the event, but their engagement with it, that contributed to development of risk awareness, and capability to seek recommended care. This ‘brokerage role’ seemed to be linked to supportive processes at the PHC facilities.
Working with provider choice: ‘you tell us to go there but they are not giving’
This theory posits that where government-run PHC facilities are equipped with medicines and are accessible to the community (C1), ASHAs will refer to these services (I), community members at high CVD risk will consider them acceptable (M) and attend for care (O). However, where other local healthcare providers have a relative advantage (more accessible, or ‘better’ ‘more trusted’ medicine supplies), (C2), community members will find them more acceptable (M) and use these other services for medical follow-up (O).
ASHAs were reluctant to refer to facilities that did not provide medications, and the high-risk cohort were reluctant to attend without assurance that sufficient supplies of medications (that being sufficient for a month) would be provided. ASHAs from PHC 2, where the facility was some distance from the select villages explained that people would not attend for follow-up care there because of both the distance required to travel, and inadequate medication supply—‘they need to spend for the charges and comeback hence they are not coming.' (ASHA FGD PHC2). This did not reflect well on the ASHAs, and so they were reluctant to refer here: ‘they come empty handed and question us, you tell us to go there and they are not giving’ (ASHA FGD PHC2).
In PHC5, while 80% of those at high CVD risk attended the PHC facility for CVD risk management at least once, the PHC facility doctor believed the community obtained their BP lowering medicines from service 104 not from the PHC pharmacy, and attendance at his facility was suboptimal because ‘they want something, medicine, lab tests, or some kind of money benefit like a cheque’… (MO IDI PHC5). Use of service 104, in preference to the government PHC facility, may have been due to medicine supplies and proximity in some cases, but in others, in response to apparent community distrust of the PHC facility, some ASHAs chose to refer their high risk patients to service 104, with demand for this service increasing almost beyond capacity ‘70 members come and in 2 hours they create havoc’ (ASHA FGD PHC3), with relatively lower use of the government PHC facility by the high-risk cohort (55% attending at least once compared with 69% overall) (table 1).
A further factor influencing utilisation of the government PHC facility for follow-up care was the availability of alternate providers, coupled with irregularities in medication supply. In PHC8, where there was no single, reliable provider of medicines, ASHAs explained that patients sourced medications from different health services depending who had supplies in any given month: ‘104 bring 1 month they do not bring another month’ (ASHA FGD PHC8) and ‘Byrraju Foundation (an Indian NGO) is there they even go there and get it, they won’t stop’ (ASHA FGD PHC8). This preparedness to source from different providers also showed a motivation for adherence on the part of community members that may have been inter-twined with characteristics of this cluster already described. This cluster showed positive intervention effects on all trial outcomes despite relatively low use of the government PHC facility for CVD risk management.
In PHC 12, while there were high levels of attendance at the government PHC facility (89% attending one or more times), effectiveness of medications supplied by the facility were disputed by some community members who reported that as the medicines were ‘not sufficient for them’ (ASHA FGD PHC12) they had changed to private sources of supply. There was an apparent contradiction in the data—on the one hand, medication availability was a driver for attendance at higher-level medical care, and on the other, community members’ distrust of the quality and ‘strength’ of government-issued medicines was raised as a serious barrier to effectiveness of the intervention by ASHAs, backed up by examples of patients refusing treatment or switching to different sources of supply because of these concerns. In seeking to understand this, we first noted that there was a general context of scepticism about the other PHC facility medications recommended by ASHAs (eg, iron tablets)—some of these would not have been recognised as having a curative purpose, and had known side effects, and as such were not popular with community members—and it is plausible that the CVD risk management medications from government sources may have become tarred with the same brush for people holding such views. This is explored further below.
Influencing community members’ attitudes to medicines: ‘why is it not showing an effect on me?’
This theory posits that where ASHAs have good relationships with local health providers who are equipped with adequate medication supplies (C), they may seek to change any negative attitudes and beliefs about government medicines, increasing acceptability of these medicines (M), leading to increased likelihood of adherence to recommended care (0).
In PHC14, a cluster where ASHAs appeared to have good relationships with a range of local health providers, and whose risk communication emphasised the importance of medical follow-up, ASHAs related community members concerns about government-issued medicines being ineffectual, ‘even when I gulp three medicines of yours, why is it not showing an effect on me?’ (ASHA FGD PHC14) or having unwanted side-effects ‘when I am using them, I am not feeling ok.’ (ASHA FGD PHC14). These ASHAs described helping patients overcome resistance to taking government-issued medications by explaining that packaging may differ, but the contents were the same as a more trusted medication. They cited instances where patients had been buying BP-lowering medications privately, and then switched to the more affordable source of supply from the government hospital. This ASHA response occurred in the context of longer duration of engagement with the intervention (being a group 1 cluster), and an engaged government hospital which had commenced routinely checking BP—a change the ASHAs attributed to the work of the intervention.
In contrast, in PHC3, a shorter duration cluster (block 3), where ASHAs also reported community resistance to government medicines, this deliberate intent to challenge community perceptions was absent—some ASHAs appeared to concur with the community perception, saying that government medicines were not working for most people, necessitating 'buying outside', and expressing disappointment that different medications were not being provided through the project. In PHC8, also shorter implementation duration, a community member described that he obtained BP control medicines from an RMP, after receiving what he perceived as inadequate dosage from the government source. ‘I took the tablets [from the Government Hospital] but felt that the dosage was not enough so I went to RMP doctor. He said I had BP of 170 and I need to stop what I was taking for a while and gave me another medicine which I bought outside. I am using them now.’ (Patient IDI PHC8). This scenario, of following ASHAs’ advice but then changing course, was described by others. The decision about what service provider to consult for CVD risk management was not made ‘once off’, but was remade on the basis of past experience and considering trade-offs—we note the relative ease of consulting RMPs who tended to be more proximate, and the additional travel time and wait time typically incurred at government health facilities and described as a disincentive by ASHAs, was income lost for many—restricting their choices over where to attend for care.
Discussion
We identified substantial variation in effectiveness of an ASHA-led DHI for people at high CVD risk, and a diversity of local contextual influences on implementation and outcomes in this single district in rural India. Our study contributes to a growing body of literature using mixed-methods process evaluations alongside cRCTs,11 20 23 and we extend this body of work through our specific focus on the local level, and through our application of a realist-informed analysis showing how local contextual factors may have influenced outcomes.
We focus here on implications for PHC service strategy interventions that entail task shifting to community health workers, and those where effectiveness of a community health worker intervention is dependent on other healthcare providers (see box 1). We also seek to connect our findings with other literature about local contextual influences on uptake of new or reconfigured free health services in LMICs.
Strategies for enhancing effectiveness of primary healthcare (PHC) service strategy interventions in different local contexts
The PHC service strategy intervention will be more effective if it:
Encourages PHC facility doctors to provide public or visible support to new roles to be undertaken by community health workers.
Identifies and recruits other respected providers of curative care in the local area, able to act as ‘champions’ for the new roles, in the event that PHC facility is less engaged.
Monitors local modifications to the intervention and their outcomes—including monitoring additional tasks that may be voluntarily taken on in response to community demands.
Develops and draws on evidence about optimal risk communication approaches in community or outreach settings, and their outcomes.
Builds tailored supportive supervision for community health workers of differing backgrounds that recognise their different ways of working with communities.
Identifies the range of local health providers from different service sectors who become engaged in the area of care addressed by the intervention, and considers its role in assessing the quality and appropriateness of care provided by these care providers for identified patients.
Supports informed decision making by patients in respect of care sought following referral.
We found that community members’ trust in ASHAs as competent to undertake their new roles was important for uptake of the new services, and that legitimacy from higher level providers, together with the DHI technology, enabled the development of trust. Trust here is defined as ‘a state of mind in which the individual expects the person with whom she interacts to react in a non-harmful or beneficial manner…’.24 The legitimacy provided by higher level providers was exemplified in PHC doctors codelivering services with ASHAs in an outreach model, and, in the absence of PHC facility doctor support, alternate respected providers of curative care verifying ASHAs as competent to perform the role. That ‘champions’ are essential to change efforts within healthcare,2 and that both local community embeddedness, and integration with PHC systems, are essential for effectiveness of community health workers, are well established. Our findings extend this knowledge by drawing attention to the need for locally respected clinical (curative) champions for the success of change efforts that entail task shifting. That the DHI altered community views of ASHAs’ skills is consistent with the notion that patients’ perceptions of providers’ technical skills are a core building block of trust.25 Our finding that experienced ASHAs extended their roles beyond protocol (eg, delivering medicines for some patients), and that this helped build acceptability and uptake of their services, is consistent with the findings of others and we echo concerns about the potential for women becoming overburdened by unrenumerated tasks in their efforts to elicit community support and work effectively.26
Acceptability as an explanatory concept in the process of making choices about healthcare, is dynamic and evolves with users’ experience—it is influenced by users’ sociocultural context and social interactions.21 That availability of medicines and accessibility was a key influence on acceptability of higher level providers, concurs with findings of others that credibility of primary providers is linked to their ability to provide curative services and adequate supplies of drugs.27 28 In our study, ‘service 104’, private providers, government hospitals and informal providers, while not directly targeted by intervention components, were nonetheless drawn into it by virtue of people seeking services from them after being identified as high risk by the ASHAs, and accessibility and capacity of higher level providers, and their access to medications needed for CVD risk management was not static. While some ASHAs engaged with dynamic and changing context and sought to direct patients to the most sustainable and appropriate provider for their circumstance, others may have been less proactive and influential. The longest period of implementation in our study (18 months) provided only limited opportunity for ASHAs and patients to experience CVD management as delivered by different local providers—acceptability of higher-level medical care would likely have evolved with use of services over time. Further in a longer implementation period, ASHAs and PHC facility doctors could have responded to users’ healthcare experiences with information and advice—as was evident in at least one of the longer-standing clusters. We note that while use of multiple healthcare providers for CVD risk management may have mitigated the impact of any deficits or erraticism in supply of BP control medicines from any single provider, use of multiple unconnected providers in our study setting meant that the integrated model envisaged by the intervention was not fully realised. This underscores the need for realism about what engagement with a single service sector (although the main government PHC sector) can achieve in mixed service environments and raises questions about how to support optimal care pathways in such contexts.
Our findings that ASHAs of differing backgrounds used different approaches to communicate risk, and may have emphasised different components of CVD risk management in response to local context raises questions about how ASHAs can be supported to communicate risk effectively—and indeed what the effects are of the prevailing approach in any local context, and the sustainability of these effects. Risk awareness as a mechanism influencing healthcare choices includes beliefs about the potential for harm of the condition, and beliefs about personal susceptibility to adverse outcomes.21 While there is established evidence from different topic areas about how to communicate risk to optimise benefit and minimise harm (eg, clear, repeated action-oriented messaging by a trusted leader; tailoring of messaging to target audience; and positively framed messaging), there appear to be few studies exploring the effects of different CVD risk communication approaches in LMICs in general and how these emerge from, and influence context.
Established principles of population screening require that accessible and effective treatment services are available to populations being screened,29and SMARTHealth India took this principle seriously in its design and in postpilot modifications made in conjunction with the district health department. Nonetheless our findings of the many inter-linked local contextual factors that limited the ability of those identified as at high risk to choose to use free healthcare for management of their CVD risk once identified, and the range of providers consulted, raises questions about the criteria that should be used to make a decision about when a local PHC system can be deemed ‘ready’ for outreach-based risk screening, such as that provided through community health workers; does a free service that is only accessible in working hours, and that needs to be visited weekly for top-up medications, classify as available to very low income wage earners who would be required to sacrifice income to access this care? Further, what responsibility do PHC service strategy interventions that include screening have in respect of the outcomes of the follow-up actions people take in response to community health worker instructions to seek medical care, especially when care is used outside of the implementing subsystem?
Limitations
Our study had strengths and limitations. First, the findings of our study are based on a fairly short period of implementation (median 12 months) and reflect the behaviour change achieved during the study period—a study of differing implementation duration may show different intervention effects, and more (or less) heterogeneity between clusters. Second, the themes we identified potentially overemphasised the perspective of ASHAs as we conducted fewer interviews with patients and PHC doctors. Third, while inductive thematic analysis was chosen as appropriate to identify unanticipated and locally specific-factors that were important to our study participants, it meant that we were unlikely to identify all possible influences on cluster-level outcomes—especially if respondents were unaware of them. These limitations were mitigated through initial qualitative data collection guided by the RE-AIM framework, and by analysis enriched by a previously developed MRT of free public healthcare seeking whose empirical foundations are derived from LMIC settings. Regarding the quantitative findings, since the baseline survey would have alerted those at high risk to pre-existing hypertension, some people may have been motivated to seek treatment before commencement of the intervention, introducing potential contamination bias. However, on the basis of the diversity of communities within clusters, and the plausible mechanism-based explanations identified, we are of the view that preintervention care seeking (if it occurred), was not an over-riding influence on the observed heterogeneity in effects. The decision not to conduct statistical tests of measures of association between quantitative measures of fidelity and outcome was taken deliberately, consistent with a complexity-informed view of programme effectiveness.30 While the diversity of local contexts and outcomes was a source of richness in the study this diversity meant that it was not possible to definitively identify commonly occurring themes associated with different trial outcomes, meaning that our findings are hypothesis generating, rather than definitive.
Conclusion
Our findings support calls for more emphasis and publication about consistency of effects that can help to interpret overall trial results, particularly those conducted in complex PHC service settings. By identifying plausible explanations for variation between clusters, we identified strategies for strengthening DHI-enabled PHC service strategy interventions in India and elsewhere.
Data availability statement
Data are available in a public, open access repository. Data are available on the Harvard Dataverse platform and can be accessed here: https://doi.org/10.7910/DVN/NSKFK2.
Ethics statements
Ethics approval
Ethical approval was granted by the Centre for Chronic Disease Control Institutional Ethics Committee and the University of Sydney Human Research Ethics Committee. The study was endorsed at the State level by the Government of Andhra Pradesh, at the district level by the West Godavari Director of Medical Services and at the village level by each Panchayat (the local governing council). Written informed consent was obtained from all participants contributing data prior to randomisation.
Acknowledgments
The authors would like to acknowledge teams (doctors, ANMs, ASHAs and other staff) in the primary healthcare facilities in West Godavari district for their support and participation in the study, field staff who dedicatedly collected periodic data and Gayatri Arani for assistance in managing qualitative data.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Handling editor Stephanie M Topp
Twitter @davidpeiris
Contributors GS, DPr and DPe conceptualised this mixed-methods study. KM, MAM and DPe designed and led qualitative data collection in the field. DPr, GS and BP led the analysis of the qualitative data and conducted the mixed-methods analysis. QL provided statistical analysis and quantitative data management. GS drafted the paper and incorporated feedback. All authors contributed to interpretation of the findings.
Funding This study was funded by an Australian National Health and Medical Research Council (NHMRC) Global Alliances for Chronic Disease Grant (ID1040147). RJ is funded through a Future Leader Fellowship by the National Heart Foundation (Grant number 102059) and the UNSW Scientia Fellowship.
Disclaimer The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests The authors have declared that no competing interests exist. The George Institute for Global Health has a wholly owned social enterprise that is conducting commercial projects that include aspects of the intervention tested in this study.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.