Abstract
The COVID-19 pandemic has swept the world and required the mobilization of scientists and clinicians around the world to combat this serious disease. Along with SARS-CoV-2 virology research, understanding of the fundamental physiological processes, molecular and cellular mechanisms and intracellular signaling pathways underlying the clinical manifestations of COVID-19 is important for effective therapy of this disease. The review describes in detail the interaction of the components of the renin-angiotensin system (RAS) and receptors of end-glycosylated products (RAGE), which plays a special role in normal lung physiology and in pathological conditions in COVID-19, including the development of acute respiratory distress syndrome and “cytokine storm”. A separate section is devoted to the latest developments aimed at correcting the dysfunction of the RAS caused by the binding of the virus to angiotensin converting enzyme 2 (ACE2)– the central element of this system. Analysis of published theoretical, clinical, and experimental data indicates the need for a complex treatment to prevent a severe course of COVID-19 using MasR agonists, blockers of the AT1R and NF-κB signaling pathway, as well as compounds with neuroprotective and neuroregenerative effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The pandemic caused by a new strain of coronavirus, to which the International Committee on Virus Taxonomy gave an official name SARS-COV-2 on February 11, 2020, has spread throughout the world due to the lack of effective methods of prevention, alleviating the course of the disease and reducing mortality [1, 2]. According to the records, at the beginning of October 2020, over 1.2 million people died in the world, and more than 35.2 million officially fell ill. The mortality rate in the USA was about 2.81% and in Russia, 1.43%. In the European countries hardest hit by the pandemic, more than 10% of patients with COVID-19 have died. The head of the World Health Organization, Tedros Adanom Ghebreyesus, believes that the spread of the pandemic in the world is accelerating globally [3, 4]. At present, a “second wave” of infection is expected and new varieties of this virus with increased virulence have appeared. Therefore, the development of vaccines and new effective pharmacological drugs for the treatment of this disease is an urgent problem.
Main symptoms of COVID-19
Previously, similar infectious diseases, called Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), occurred in 2002–2003 and in 2011. These diseases were caused by other strains of coronaviruses – SARS-CoV and MERS-CoV, respectively. SARS-CoV-2 has a high similarity (79%) to the SARS pathogen. The most common clinical manifestation of a new variant of coronavirus infection is bilateral pneumonia; in 3–4% of patients an acute respiratory distress syndrome develops, which in 75% of these patients is accompanied by thrombotic coagulopathy. In the terminal stages, thrombosis and “cytokine storm” are developing, characterized by systemic release of pro-inflammatory cytokines: interleukins IL-2, IL-6, IL-7, granulocyte colony-stimulating factor, chemokine 10 (CXCL10), chemokine ligand 2 (CCL2), tumor necrosis factor-α (TNF-α); and leukopenia, indicating reduced cellular immunity [5, 6]. Changes in smell (hyposmia) or deterioration in taste are observed in 15–30% of patients at an early stage of the disease and can be one of the diagnostic signs of COVID-19 [7, 8].
Brief characteristics of SARS-CoV-2
Novel coronavirus SARS-CoV-2 is a single-stranded RNA virus that belongs to the Coronaviridae family, line Beta-CoV B. It is highly contagious and is mainly spread by people with asymptomatic course, accounting for more than 30% among those with a positive test for the presence of a virus in the body [9].
The main initial target of the virus is angiotensin-converting enzyme 2 (ACE2), localized on the outer membrane of cells in many organs and tissues [10–12], although the possibility of its interaction with other receptors, such as CD147, CD26, DPP4, and TMPRSS2, cannot be completely ruled out [13–15].
To enter into the cell, SARS-CoV-2, like the SARS-CoV, uses the spike (S) protein, consisting of two subunits [16]. Unit S1 binds to the N-terminus of the extracellular domain of the ACE2 receptor. Unit S2 is required for the activation of endocytosis and the virus entry into the host cell. In lungs, SARS-CoV-2 preferentially interacts with type II pneumocytes, constituting less than 10% of alveolar cells. Type II pneumocytes coexpress, along with ACE2, the serine protease TMPRSS2, which binds with S1/S2 cleavage site of the S protein, priming the second furin-like cleavage site S2′ located on the S2 subunit and directly requiring a host furin protease, highly expressed in lungs. Thus, the S protein must be cleaved at both S1/S2 and S2′ cleavage sites for virus entry into the cell. It should be noted that the activation of S2′ site is a distinctive feature of SARS-CoV-2 [17, 18]. Currently, the crystal structure of the ACE2 molecule and the binding site with SARS-CoV-2 has been determined [12]. In most cases, ACE2 exists as a heterodimer complex with the intracellular membrane amino acid transporter BAT1. Two such complexes, in turn, form a homodimer due to interactions of the similar regions of two ACE2 molecules. The S1 unit of spike protein of SARS-CoV-2 recognises and attaches to this homodimer on the cell surface due to polar interactions. It is important to note that this region is not associated with protease activity (protease domain) of ACE2, which is responsible for the removal of one amino acid residue from angiotensin II (ANGII, ANG1-8), the peptide regulator, in the renin-angiotensin system (RAS). A trimer of viral proteins can be bound to one ACE2, and two trimers, to the dimer of ACE2, respectively [12]. Initially, the virus infects the superficial goblet cells in the mucosa of the nasal cavity, tongue, larynx, and lungs, where it specifically infects type II pneumocytes–the cells secreting surfactant, which prevents adhesion of alveoli and exerts a significant antimicrobial effect [19, 20]. It is noteworthy that the activity of proteases promoting the virus penetration into the host cell decreases with an increase of pH in the extracellular and intracellular environment. This, for example, is achieved by treatment with hydroxychloroquine or chloroquine [21]. Therefore, during the COVID-19 pandemic, in the absence of effective antiviral drugs and vaccines, inhalation of a 1% sodium bicarbonate is advisable as a preventive tool. This method, without thinking about the mechanisms of its action, has long been used for various respiratory and pulmonary infections and influenza, empirically proving its effectiveness [22]. Numerous ACE2 receptors are also present on vascular endothelial cells of the heart and kidneys, on neurons and glia, as well as on enterocytes lining the small intestine and involved in the process of nutrient absorption. The increase in the permeability of the intestinal wall as a result of inflammation caused by the virus is accompanied by changes in the microbiota and the entry of viral particles and toxins into the bloodstream [23].
Currently, direct evidence of the presence of SARS-CoV-2 in the human brain has already been obtained [20]. Dissemination of the virus into the brain can occur both from the systemic circulation as a result of a violation of the blood–brain barrier, and directly from the nasal and oral cavity along the olfactory and glossopharyngeal nerves, as well as along the branches of the facial and vagus nerves. The penetration of the virus into the brain, rich in ACE2 receptors located on the membranes of neurons and glia, can lead to impaired brain function [20, 24, 25]. Earlier, in experiments on transgenic mice carrying the human ACE2 gene, it was established that the SARS-CoV virus can penetrate the brain, damage neurons in many brain areas, including the respiratory center located in the medulla oblongata. The cause of death of these animals was pathology, similar to acute respiratory distress syndrome, which develops in severe COVID-19 in humans [26]. In this regard, it is important to note that impaired respiratory function in COVID-19 can be both a consequence of a local disturbance of gas exchange in the alveoli of the lungs, and a disruption of the respiratory center in the brain at the later stages of infection [27–29]. The regulation of the activity of inspiratory neurons in the respiratory center is mainly carried out by chemoreceptors, which are very sensitive to an increase in the blood level of CO2 and H+ and, to a much lesser extent, to the O2 level. These factors enhance the activity of the respiratory center, affecting the central and peripheral chemoreceptors. This circumstance must be taken into account both when applying artificial lung ventilation (ALV) and on weaning from ALV of the COVID-19 patients. Observations of patients with COVID-19 indicate the development of neurological and psychiatric disturbances in the form of strokes, polyneuropathies, depression, and the consequences of post-traumatic stress. It has been suggested that neurodegenerative diseases, including Parkinson’s disease and Alzheimer’s disease may develop in patients with COVID-19 [30–32]. It has now been established that the penetration of the virus into the cell causes an increase in the expression of genes responsible for the synthesis of proteins associated with apoptosis [33], which leads to the death of the infected cell. Previously, we found that damage of the olfactory bulb, the first brain structure associated with the olfactory nerve, can actually induce the Alzheimer’s type neurodegeneration in animals, and intranasal administration of neuroprotective agents, such as heat shock protein or YB-1, can prevent neuronal death and protect the brain from the development of degeneration [34–36].
It is known that the biology of the virus is largely dependent on the host organism. As mentioned above, for penetration into the cell and replication, SARS-CoV-2 uses a number of host proteases, including cathepsin L, cathepsin B, trypsin, factor X, elastase, furin, and TMPRSS2 (transmembrane serine protease 2); at the same time, it was found that the binding of the virus to ACE2 activates the expression of this enzyme, which plays an essential role in protecting lung tissue from damage [37–39]. Therefore, inhibition of the synthesis of viral proteins or impacts on the mechanisms of viral replication through the blockade of endogenous proteases can harm the host organism by severe side reactions, which are produced by a number of pharmacological agents [40–49]. Currently, the most effective drug for the treatment of COVID-19, according to WHO, is Remdesivir, created in the United States, which blocks viral RNA synthesis by inhibiting viral RNA polymerase, competing for inclusion in its molecule with endogenous ATP [50, 51]. Currently, the main focus is on the development of vaccines, but one should keep in mind that the vaccine, being specific for this virus, may not be effective in preventing infection with other viral infections, even related to SARS-CoV-19. Good results are obtained by the method used in the last century– injection of blood serum from people who have had an infection [52]. Unfortunately, only 30% of such serum has a sufficient titer of antiviral antibodies (IgG) required for a therapeutic effect. In a significant part of carriers of a viral infection, the disease is asymptomatic, which indicates the presence of protective mechanisms in the host’s organism, the activation of which is capable of leveling the manifestations of the disease, but not getting rid of the virus. Although it is assumed that such a protective function is primarily performed by nonspecific innate immunity [53], nevertheless, the data suggesting an important role of the RAS state have been obtained recently [23, 38, 39, 54]. However, there are practically no drugs that affect ACE2–the central receptor for the binding of the virus and the main regulator of the RAS. Even the possibility of using angiotensin receptor 1 (AT1R) blockers in patients with COVID-19 with hypertension has caused heated discussions among physicians [55–57].
We tried to fill in the missing information with new data on the inclusion of RAS in the pathogenesis of COVID-19 and possible targets of therapeutic action.
Physiology of RAS
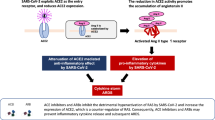
It has now been established that the risk of a severe course of COVID-19 is not so much the patient’s age itself as the so-called age-related diseases, such as hypertension, diabetes, cardiovascular pathologies, in the development of which the RAS and the ACE2 receptor are directly or indirectly involved [23]. It has been known for over 20 years that ACE2 is one of the most important elements of the RAS, which maintains homeostasis by regulating the interaction of the cardiovascular and respiratory systems, electrolyte balance, carbohydrate metabolism, and blood pressure regulation [23]. Figure 1 shows the main elements of the RAS and their regulators in a healthy and SARS-CoV-19-infected organism.
The main components of the RAS. Left, the first branch of the RAS; right, the second compensatory branch of the RAS. Rectangles are biologically active angiotensins and their receptors; ovals are enzymes that convert them. AGT, angiotensinogen, serum globulin, a precursor of ANG I. ACE2, angiotensin-converting enzyme 2, the central regulator of the balance of the activity of the branches of the RAS, interacting with SARS-CoV-2 in COVID-19.
Classical RAS has two branches, one of which includes angiotensinogen/renin/ANGI (ANG1-10)/ angiotensin converting enzyme (ACE)/angiotensin II (ANGII, ANG1-8)/angiotensin receptor type I (AT1R). Normally, this branch activates the central and peripheral mechanisms responsible for the regulation of blood pressure. However, it is precisely its activity that is associated with the development of various pathologies, including fibrosis, inflammation, cardiovascular pathology, metabolic syndrome, cancer, aging, diabetes, and hypertension [58]. It was found that ANGII, interacting with AT1R, activates ERK1/2 (kinases involved in the transduction of extracellular signals) and p38 MAPK signaling pathways, which leads to a decrease in the expression of the ACE2 gene [59] and an increase of the ADAM17 activity by phosphorylation of its intracellular domain [60, 61]. The activation of ADAM17 is also associated with the emergence of a circulating form of TNF-α followed by the induction of inflammation [23, 62]. While the first branch of the RAS has been known for more than 100 years, the second branch, including ACE2/ANG1-7/MasR, was discovered quite recently, and some aspects of its functioning are still not fully investigated [63].
Activation of the other branch of the RAS has an effect opposite to the first branch of the RAS and is accompanied by a decrease in blood pressure, anti-inflammatory reactions, activation of innate immunity, and stimulation of cell differentiation. ACE2 is a key enzyme that converts ANGII (ANG1-8)–the ligand of AT1R, into ANG1-7 with subsequent activation of MasR [64]. It should be noted that there is also a minor shunting pathway that reduces hyperactivation of the first branch of the RAS, through the binding of ANGII to the second type angiotensin receptor (AT2R), which mediates a decrease in blood pressure and vascular dilation. However, the density of this receptor is significantly lower than that of AT1R and sharply decreases with age. Considering all of the above, special care should be taken in the development of drugs against SARS-CoV-2, blocking the protease domain of ACE2, which can significantly reduce the effectiveness of the “protective” role of the second branch of the RAS (ACE2/ANG1-7/MasR) and dramatically increase binding of ANGII to AT1R, causing an exacerbation of the course of all comorbidities. On the other hand, blocking the ACE2 binding site on the spike protein of the virus itself can provoke allosteric modifications, which provide a new possibility for the binding of the viral S protein to ACE2. Some authors foresee the possibility of a single mutation in the S protein at position 501, which will lead to an increase in the ability of the virus to bind to human ACE2 with poorly predictable consequences [16].
As mentioned above, ACE2 is widely distributed in the body and can exist in two forms–predominantly membrane-bound monocarboxypeptidase (120 kDa) and as a circulating free form consisting of one extracellular domain bearing a binding site for ANGII and an interaction site with the viral S protein. Protease ADAM17 is involved in the formation of the free form. It is important to note that knockout mice ACE2–/– exhibited a number of features characteristic of COVID-19: oxidative stress, neutrophilic infiltration, release of proinflammatory cytokines, activation of mitogen activated protein kinase (MAPK) [65]. Apparently, not the virus itself but virus-induced imbalance in the activity of the two branches of the RAS may be one of the basic mechanisms of the disease progression. Currently, there is a fierce debate about what really happens with the ACE2 receptor in patients with COVID-19. In experiments on animals infected with the virus, a decrease in the activity of this receptor was revealed mainly in the lungs, but not in the surrounding tissues, which was also accompanied by a decrease in the content of ANG1–7 and an increase in the activity of ANGII with all the negative consequences of its activation [23]. A moderate decrease in the expression of the ACE2 gene is also observed in human lung cells infected with the virus [66]. One of the factors of greater susceptibility to infection and a more severe course of the disease in elderly people with concomitant pathologies may be a reduced level of this receptor in various organs and tissues [67, 68]. However, in patients who underwent COVID-19, there was no drop in the level of this receptor in the lungs, and in studies on tissues and cells of non-human primates and human cell culture, it was found that under the influence of viral infections, not a decrease but, on the contrary, an increase in the expression of the ACE2 gene occurs. The classical scheme for the formation of the body’s response to the introduction of the virus includes several stages. First, viral RNA is recognized by endosomal RNA receptors of the host, TLR3 and TLR7, as well as by the cytosolic RNA sensor, RIG-I/MDA5, and the signaling cascades NF-κB and IRF3 are activated with the translocation of these transcription factors into the nucleus, stimulating the expression of type 1 interferons (alpha and gamma interferons) and their release into the extracellular environment. Further, interferons interact with membrane receptors IFNAR and as a result, the JAK-STAT system is activated. Proteins STAT1 and STAT2, phosphorylated with the participation of JAK1 and TYK2 kinases, form a complex with IRF9 and enter the nucleus, where they stimulate certain genes (ISGs), while the response is under the control of a special element ISRE located on the promoter of these genes. It is assumed that the element sensitive to the stimulatory effect of interferons is also located on the promoter of the human ACE2 gene [54]. However, it has been established that coronaviruses interfere with the formation of the body’s defense reaction both at the stage of interferon synthesis and at the stage of STAT1 phosphorylation. The initially delayed stimulation of ISGs is then realized in the hyperactivation of the expression of proinflammatory cytokines [69]. Perhaps that is why the increased use of interferons in the late phase of infection may be accompanied by both an improvement and worsening of the disease course [54, 70, 71]. An overload of the activated interferon system can indeed lead to a “cytokine storm” and the need for the use of immune system suppressors [72], including antibodies to proinflammatory cytokines, such as IL-6 and TNF-α or their receptors [40]. It is noteworthy that although mouse ACE2 exhibits 84% identity with human ACE2 and, as in humans, is predominantly expressed in the lungs, heart and kidneys [73], mice are not infected by SARS-CoV-19 and activation of the ACE2 gene is not regulated by interferons. These facts imply that the extrapolation of data obtained on non-transgenic mouse cell models to humans should be treated with caution [54].
ANG1–7 is the next component of the second branch of RAS, which was first discovered in the rat brain in 1983 [74], but its importance was proved only in 1988 due to the presence of hypotensive and antiproliferative effects, which was opposite to the action of ANGII [75, 76]. Basically, ANG1–7 is converted from ANGII by ACE2, since the affinity of ACE2 for ANGII is 400 times higher than for other angiotensins [77]. However, at least two more minor pathways of ANG1–7 formation have been discovered, including ANG1/ANG2/ANG1–9 /ACE /neprilisin/ANG1–7 and ANGI/neprilisin/ANG1–7 [78]. In 2000, the effect of ANG1–7 on Na+,K+-ATPase activity was discovered, which at high concentrations of ANG1–7 is similar to the action of ANGII and, apparently, is mediated through the A779-sensitive receptor but not through MasR [79, 80]. Changes in the level of circulating ANG1–7 are associated with chronic severe diseases such as hypertension, preeclampsia, and myocardial diseases, including myocardial infarction, renal disease, and liver cirrhosis [77]. It was also found that ANG1–7 lowers glucose tolerance and enhances insulin sensitivity, and MasR receptor is considered as a component of the insulin receptor signaling pathway [81]. In experiments on mice with cerebral ischemia, ANG1–7 suppressed the inflammatory response through inhibition of the NF-κB pathway [82]. The effects of ANG1–7 include inhibition of noradrenergic neurotransmission, inhibition of apoptosis, enhancement of cell differentiation, and antioxidant action. Activation of the ANG1–7/MasR system is recommended for stroke treatment [83]. It is interesting to note that an increase in the ratio of ACE/ACE2 levels was accompanied by acute respiratory distress syndrome, which could be prevented with the use of ANG1–7 or AT1R blockers [84]. The ability of this peptide to inhibit apoptosis of alveolar epithelial cells is also important for the treatment of COVID-19 [85]. In the brain, ANG1–7 has a neuroprotective effect and stimulates synaptogenesis [86]. The restoration of smell in people who have recovered from COVID-19 may be due to the activation of neurogenesis and differentiation of neuronal progenitors migrating from the subventricular zone to the olfactory bulb [87]. However, the wide use of ANG1–7 in the clinical practice is limited by its rapid destruction in the body and, possibly, by the complexity of its delivery into the lung and brain tissue after its intravenous administration.
The action of ANG1–7 is mediated through interaction with MasR, which was first described in 2003 [63]. After binding to the ligand, MasR is internalized into early endosomes through a clathrin-mediated mechanism, and then re-incorporated into the cell membrane. MasR is a classic heptaspiral receptor coupled to a heterotrimeric Gαq/11 protein. Its interaction with the ligand leads to the activation of phospholipase C. It was found that activation of MasR has opposite effects with AT1R, with which MasR forms a heterodimer and, thus, blocks its activity [88]. MasR activation reduces the expression of inflammatory genes and has a protective effect on endothelial cells, neurons, and the blood–brain barrier. Binding of ANG1–7 to MasR stimulates the formation of nitric oxide (NO), synthesis of arachidonic acid, Akt, and phosphorylated ERK1/2. Recently, evidence has emerged that MasR can be activated not only by ANG1–7, but also by other compounds, such as neuropeptide FF, alamandin, angiotensin III, angiotensin IV, and angioprotectin. MasR agonists have a positive effect in the treatment of chronic renal failure by arresting the development of fibrosis and inflammation in the kidneys [89]. It is of note that all RAS receptors, including ACE2, are located not only on the outer membrane of cells, but also have mitochondrial and nuclear localization [90]. It remains unclear, whether the virus interacts with intracellular ACE2.
From the presented data it follows that MasR agonists can neutralize the disorders in RAS caused by a decrease in the level of the ACE2 receptor in COVID-19, restore the balance in the activity of the two branches of the RAS, and therefore represent promising compounds for the development of treatments for this disease.
Promising Developments of MasR ligands
Currently, along with ANG1–7, other peptide and inorganic ligands of MasR are known. Thus, [Ala1]-ANG1–7 is recognized by MasR and causes vasodilation of blood vessels, [Pro1, Glu2]-ANGII is present in human blood plasma and is characterized by high affinity for MasR [63, 90]. D-Ala7 ANG1–7 and D‑Pro7 ANG1–7 are MasR antagonists and completely block the activity of ANG1–7 [63, 91]. At the disposal of researchers there are also non-peptide agonists of this receptor, such as AVE 0991 and AR234960, which have anti-inflammatory and anti-apoptotic effects and also reduce oxidative stress through activation of the MasR/PKA/CREB/UCP-2 signaling pathway [92]. A disadvantage of such agonists as AR234960 and NPFF neuropeptide is a rapid desensitization of MasR and the accumulation of intracellular calcium [93]. For the treatment of cognitive impairment and memory loss associated with neuroinflammation, a PNA5 agonist has been developed, which is a glycosylated ANG1–7 with the substitution of serine in the seventh position of the amino acid residue proline with the addition of glucose and the formation of amides at the C-terminus, which suppresses the oxidative burst in the endothelial cells via activation of MasR. This compound is characterized by improved bioavailability due to increased permeability of the blood–brain barrier, which is accompanied by increased expression and activation of MasR in the brain [94]. High activation of MasR was observed upon binding to monomers FLGYCIYLNRKRRGDPAFKRRLRD (CGEN-856S) and SMCHRWSRAVLFPAAHRP (CGEN-857S), in which the cysteine amino acid residue is replaced by serine [95]. An increase in the MasR expression in the lungs is caused by Liraglutide, an agonist of the glucagon-like peptide 1 receptor [96]. Currently, NCT01597635, a recombinant extracellular domain of human ACE2, which stimulates the expression of MasR and simultaneously, through interactions with the virus, reduces its ability to bind to the membrane ACE2 and infect cells, is currently in the second phase of clinical trials as a drug against COVID-19 [97].
Thus, for the treatment of COVID-19 and other viral infections, the causative agents of which use ACE2, for the correction of RAS disorders, preference should be given to persistent analogs of ANG1–7, activating MasR, or stimulation of minor pathways, where the central agent is neprilisin (Fig. 1).
Local and systemic RAS
Although previously RAS was characterized as an integrated endocrine system, the targets of which are both central and peripheral receptors, now there is convincing evidence for the presence of RAS in individual organs and tissues, where it affects the local activity of cells and is represented by intracellular and extracellular components [98, 99]. In particular, in the lung tissue a very important role belongs to the components of the second branch of the RAS, ACE2/ANG1–7/MasR, which have a protective effect on the lungs against various damaging factors [100]. Normally, these local systems function relatively independently, which is indirectly evidenced by the differences in the content of RAS components, from femptomolar to nanomolar concentration in different organs and extremely low levels of ACE2 or ANG1–7 in the blood [101, 102]. An increased systemic content of free extramembrane form of ACE2 is noted in chronic patients with deterioration of their condition, especially in terminal conditions [99, 103].
The data indicating that when MasR, AT1R, or AT2R agonists were injected into the renal artery, only changes in kidney function were noted, which did not affect cardiac activity or blood pressure, confirm the relative independence of the functioning of local RAS [104]. This circumstance must be taken into account when using systemic administration of drugs designed to affect the components of local RAS.
As already indicated, local RAS are involved in maintaining tissue homeostasis and tissue regeneration after injury. It has been shown that disorders in the local RAS lead to such dysfunctions as atherosclerosis, cardiac hypertrophy, renal fibrosis, type II diabetes, insulin resistance, and obesity [105]. Moreover, the expression of the RAS components in such pathologies most often increases: the level of angiotensinogen, the activity of ACE, and the level of ANGII increase [106]. Therefore, inhibitors of ACE or AT1R have shown their effectiveness and are very widespread in the treatment of hypertension, heart hypertrophy, myocardial infarction, chronic heart failure, diabetic nephropathy, and pulmonary pathology. The imbalance of the local RAS, expressed in an increase in the ANGII/ANG1–7 ratio, plays a special role in the lungs, causing acute respiratory distress syndrome with 30% mortality. The increased local level of ANGII in the lungs enhances the infiltration of immune cells, which in turn increase the synthesis of proinflammatory cytokines TNF-α, IL-1, IL-6. It is important to note that the binding of ANGII to AT1R, as well as the interaction of a number of proinflammatory cytokines with their receptors, activates JAK/STAT signaling pathways [107, 108].
As the disease progresses, endothelial cells of microvessels are affected not only in the lungs, but also in the heart, kidneys and intestines. Binding of ANGII to AT1R leads to the activation of p38 MAPK and NF-κB signaling pathways, along with hyperactivation of the immune system, which causes a “cytokine storm” [109]. Apparently, a similar development of events beginning in the lungs occurs in patients with COVID-19. Therefore, the development of JAK blockers (IJAK), which simultaneously prevent the development of the effects of activation of AT1R and a number of pro-inflammatory cytokines, is promising.
The data on the influence of various antihypertensive drugs on the content of RAS components indicate that the most effective means of increasing ANG1–7 in blood plasma is a recombinant form of free ACE2 (Table 1) [110]. It is of note, however, that this compound has not passed clinical trials, because despite the expected biochemical changes, a decrease in blood pressure was observed only in patients with an initially elevated plasma ANGII content, and the effect was achieved only at very high concentrations of ACE2 [97].
Reflecting on the biology of the SARS-CoV and SARS-CoV-19 viruses, which are characterized by low contagiousness relative to other viral infections, but increased affinity for ACE2, and given a significant number of cases with asymptomatic course of infection, it can be assumed that it is not the virus itself, but the body’s response to its presence that determines the nature and severity of the course of the disease. It should be noted that the virus infects easily accessible large glandular cells located in the respiratory tract, with a well-developed system for the synthesis of a variety of compounds that make up the secretion they produce. The violation of secretion from the onset of the disease is evidenced by the practically absence of a runny nose and a dry cough. Subsequently, alveolar breathing is impaired, probably owing to alveolar adhesion agglutination caused by insufficiency of the surfactant secreted by type II pneumocytes and involved in gas exchange, inflammation and activation of the bacterial flora always present in the respiratory tract, as well as impaired water-salt metabolism, infiltration of neutrophils, etc. A decrease in the number of ACE2 receptors as a result of internalization of their complex with the virus would theoretically prevent further spread of infection in the host. But when the virus enters the cell, stimulation of the expression of the ACE2 gene is noted with a delayed moderate activation of the synthesis of interferons – elements of innate immunity, along with the appearance of T-lymphocytes and activated macrophages. Apparently, the enhanced synthesis of ACE2 compensates for its decline and has a less dramatic effect on the second branch of the RAS and the level of ANG1–7. The process can occur locally in the lung tissue and, possibly, in the brain, limited to the olfactory bulb, which is a powerful barrier on the way of further penetration of viruses, microbes, and other harmful compounds into various brain areas [88]. The preservation of membrane ACE2 is also favored by the possibility of the spread of SARS-CoV-2 by fusion of affected cells with healthy ones and the syncytium formation, due to the interaction of furin protease with a site on the S2 subunit of the virus S protein [17]. Apparently, these mechanisms allow the infection to proceed locally and asymptomatically for a long time without affecting other systems of the body, where ACE2 receptors are found even in greater quantities compared to the lungs: heart, blood vessels, kidneys, intestines, and brain neurons. However, in elderly people with concurrent diseases and with reduced content of ACE2 in the lungs, not only relatively few type II pneumocytes (less than 3.6%) and ciliary cells (about 8%) coexpressing ACE2 and TMPRSS2 are affected, but also neighboring pneumocytes of types I and II carrying single ACE2 receptors [19]. Clinical data also indicate the formation of syncytium [14]. This unfavorable scenario quickly leads to the development of acute respiratory distress syndrome, characterized by severe inflammation, oxidative stress and edema, indicating an imbalance in fluid balance in the lungs. It is unlikely that the reason for such a serious complication is only a violation of the release of surfactant by type II pneumocytes, since the virus infects, as mentioned above, a relatively small number of these cells, and serious damage to the lung tissue is noted even in persons with a relatively mild course of the disease. In this connection, it was suggested that in COVID-19 not only the activity of the second compensatory branch of the RAS is weakened, but as a result of the activation of AT1R, an additional mechanism is involved, mediated through the receptor for advanced glycation endproducts (RAGE), which is involved in the development of acute respiratory distress syndrome [111].
The Role of RAGE in the Pathogenesis of COVID-19
It is known that RAGE is a multifunctional receptor with numerous ligands that have binding sites on both the extracellular and intracellular domains [112, 113]. RAGE is involved in the pathogenesis of many severe chronic diseases, including pulmonary pathologies associated with fluid imbalance and inflammation [114–116]. In the lungs, RAGE is localized on numerous alveolar pneumocytes of type I (95–98% of all alveolar cells), responsible for gas exchange between air in the alveoli and blood in pulmonary capillaries [117]; besides, RAGE also is found on the membranes of type II pneumocytes [118]. In a young healthy organism, the background expression of RAGE is negligible, with the exception of lung tissue, where this receptor is responsible for the balance of ions in the tissue fluid and for the regulation of the redox potential. It is known that the formation of disulfides occurs through the oxidation of free thiols (for example, SH groups). Thioredoxin 1 reduces the disulfide bonds of proteins in the reaction of thiol–disulfide exchange, while the thiol groups of thioredoxin are oxidized, and their reduction occurs through a redox reaction with thioredoxin reductase. The cycle of oxidation and subsequent reduction serves as a molecular switch to regulate the physiological function of proteins and to maintain homeostasis, and disruption of this process leads to oxidative stress [119]. Experiments on alveolar epithelial cells showed that binding of RAGE to ligands was accompanied by increased protein oxidation, while siRNA-induced RAGE knockdown led to an increase in free SH groups and a decrease in the expression of antioxidant enzymes, such as superoxide dismutase 1, thioredoxin, and protein 17 containing the thioredoxin domain [120]. Interestingly, compounds with a disulfide bond exerted a positive effect on models of lung damage in rodents; these compounds decreased the intensity of oxidative stress and the severity of the inflammatory reaction [121, 122]. Currently, inhalations with sodium thiosulfate (Na2S2O3) have been proposed for the treatment of affected lungs in COVID-19 [123]. In COVID-19, even with a local lesion of the RAS in the lungs, an increase in the level of ANG II and activation of the AT1R receptor is observed, followed by transactivation of the RAGE cytosolic domain and activation of the NF-κB signaling pathway leading to the expression of proinflammatory genes [124], AT1R and RAGE genes.
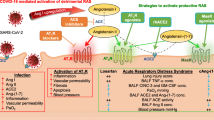
With the progression of the inflammatory process and destruction of the epithelial barrier in the alveoli, increased expression of RAGE is observed in endothelial cells and vascular smooth muscle cells, which is accompanied by an increase in their permeability [125, 126]. For numerous endothelial cells, a slightly different mechanism of RAGE activation in the interaction of ANG II with ATIR has been described, which additionally includes the expression of the gene of one of the main endogenous ligands of RAGE, high mobility group box 1 (HMGB1). Being a non-histone nuclear protein, predominantly localized in the nucleus, it can be released into the extracellular space and interact with RAGE, restarting the activation of the NF-κB signaling pathway, realizing the sequential activation scheme: ANGII/ATIR/NF-κB/HMGB1/RAGE [127]. Due to the presence of a positive feedback between AT1R activity, RAGE expression and its activation, further development of the local pathological process occurs with the transition to the systemic level and damage to the vessels of the heart, kidneys, and intestinal tract. It is important to note that the central role in the pathological relationship between AT1R and RAGE belongs to the NF-κB signaling pathway [128], the blockade of the activity of elements of which is possible using inhibitors of adapter proteins by stabilizing protein complexes to keep them in the cytoplasm, as well as through inhibition of the transcription factor, which in the experiment has a pronounced therapeutic effect [129]. Figure 2 shows the ways of possible interaction of RAS and RAGE, which should be taken into account when developing approaches to treatment of COVID-19.
Scheme of the possible interaction of the RAS and RAGE signaling pathways. Binding of the virus to ACE2 leads to an increase in the level of ANGII, its interaction with AT1R, followed by transactivation of the RAGE cytosolic domain and activation of the NF-κB signaling pathway, which stimulates the expression of pro-inflammatory genes, including RAGE and one of the central RAGE ligands, HMGB1; after exiting the cell HMGB1 interacts with the RAGE extracellular domain and restarts the process mediated by NF-κB activation.
Currently, there is no explanation yet why children rarely get sick with COVID-19, but if the disease still develops (it has received a special name Pediatric Multisystem Inflammatory Syndrome (PMIS), potentially associated with COVID-19), then it has symptoms similar to Kawasaki disease, in which endothelial cells of the vessels of the heart and intestinal tract die. In a rodent model of this disease, it was revealed that apoptosis and necrosis of these cells is caused by activation of the HMGB1/RAGE/cathepsin B signaling pathway [130].
In conclusion, the progression of COVID-19 is due to the transition from a local disorder of the RAS in the lungs to its systemic imbalance with hyperactivation of the first branch and the formation of ANGII, AT1R, and RAGE. Many authors indicate that activation of ACE2 synthesis can, on the one hand, cause activation of ANG1-7/MasR, but on the other hand, simultaneously increase the ability of the virus to enter cells. It seems that only the activation of the local ANG1–7/MasR system, along with the blockade of AT1R and NF-κB signaling pathways, will restore the balance of the RAS and prevent the negative scenario of COVID-19.
CONCLUSIONS
The review presents various mechanisms of SARS-CoV-2 infection with an emphasis on the effect of the virus on the functioning of the renin-angiotensin system (RAS), the most important system in the body involved in the regulation of the activity of the heart, blood vessels, brain, kidneys, intestines, and especially of the lungs. An imbalance in the RAS causes acute respiratory distress syndrome and cytokine storms, which are hallmarks of severe course of COVID-19. The use of pharmacological substances that repair the RAS is as promising as targeting at virus itself. However, to normalize the RAS, a more complex effect is required, including the activation of MasR along with the blockade of the AT1R and NF-κB signaling pathway. It is important to note that such a treatment, based on the knowledge of the molecular and cellular mechanisms of the disease, is applicable to any other viruses that use the ACE2 receptor for their entry into the cell. The review deliberately did not cover the issue of the role of the immune system in the pathogenesis of this viral infection, although there are a number of excellent reviews devoted to this issue [131, 132]. The therapeutic effect on a viral infection can have an unpredictable impact on the response of the patient’s impaired immune system, which determines the severity of COVID-19, which is important to consider when developing vaccines and conducting immunotherapy. Obviously, the discovery of the mechanisms of asymptomatic carriage of the virus and the correction of the body’s response to the virus will not only help to cope with this pandemic, but will also be effective in other infectious diseases.
REFERENCES
Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. 2020. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 395, 565–574. https://doi.org/10.1016/S0140-6736(20)30251-8
Paules C.I., Marston H.D., Fauci A.S. 2020. Coronavirus infections – more than just the common cold. JAMA. 323 (8), 707–708. https://doi.org/10.1001/jama.2020.0757
Data on infected with coronavirus at the beginning of October 2020. https://www.google.com/search?client= firefox-b-d&q= statistics on coronavirus in the world
El-Aziz T.M.A., Stockand J.D. 2020. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2) – an update on the status. Infect. Genet. Evol. 83, 104327. https://doi.org/10.1016/j.meegid.2020.104327
Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 395, 497–506.
Barrett C.D., Moore H.B., Moore E.E., McIntyre R.C., Moore P.K., Burke J., Hua F., Apgar J., Talmor D.S., Sauaia A., Liptzin D.R., Veress L.A., Yaffe M.B. 2020. Fibrinolytic therapy for refractory COVID-19 acute respiratory distress syndrome: Scientific rationale and review. Res. Pract. Thromb. Haemost. 4 (4), 524–531. https://doi.org/10.1002/rth2.12357
Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. 2020. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 77 (6), 1–9. https://doi.org/10.1001/jamaneurol.1127.
Gelardi M., Trecca E., Cassano M., Ciprandi G. 2020. Smell and taste dysfunction during the COVID-19 outbreak: A preliminary report. Acta Biomed. 91 (2), 230–231. https://doi.org/10.23750/abm.v91i2.9524
Qiu J. 2020. Covert coronavirus infections could be seeding new outbreaks. Nature. 20. https://doi.org/10.1038/d41586-020-00822-x
Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 579, 270–273. https://doi.org/10.1038/s41586-020-2012-7
Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically-proven protease inhibitor. Cell. 181 (2), 271–280. https://doi.org/10.1016/j.cell.2020.02.052
Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. 2020. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 367 (6483), 1260–1263. https://doi.org/10.1126/science.abb2507
Li Y., Zhang Z., Yang L., Lian X., Xie Y., Li S., Xin S., Cao P., Lu J. 2020. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. iScience. 23 (6), 101160. https://doi.org/10.1016/j.isci.2020.101160
Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T., Winter H., Meister M., Veith C., Boots A.W., Hennig B.P., Kreuter M., Conrad C., Eils E. 2020. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 39 (10), e105114. https://doi.org/10.15252/embj.20105114
Vankadari N., Wilce J.A. 2020. Emerging WuHan (COVID-19) coronavirus: Glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microb. Infect. 9, 601–604. https://doi.org/10.1080/22221751.2020.1739565
Wan Y., Shang J., Graham R., Baric R.S., Li F. 2020. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 94 (7), e00127-20. https://doi.org/10.1128/JVI.00127-20
Hoffmann M., Kleine-Weber H., Pöhlmann S. 2020. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell. 78 (4), 779–784. https://doi.org/10.1016/j.molcel.2020.04.022
Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. 2020. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 176, 104742. https://doi.org/10.1016/j.antiviral.104742.
García-Fojeda B., González-Carnicero Z., de Lorenzo A., Minutti C.M., de Tapia L, Euba B., Iglesias-Ceacero A., Castillo-Lluva S., Garmendia J., Casals C. 2019. Lung surfactant lipids provide immune protection against haemophilus influenzae respiratory infection. Front. Immunol. 10, 458. https://doi.org/10.3389/fimmu.2019.00458
Buzhdygan T.P., DeOre B.J., Baldwin-Leclair A., McGary H., Razmpour R, Galie P.A., Potula R., Andrews A.M., Ramirez S.H. 2020. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in vitro models of the human blood–brain barrier. Neurobiol Dis. 146, 105 131. https://doi.org/10.1016/j.nbd.2020.105131
Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. 2005. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2, 69. https://doi.org/10.1186/1743-422X-2-69
Dobay O., Laub K., Stercz B., Kéri A., Balázs B., Tóthpál A., Kardos S., Jaikumpun P., Ruksakiet K. Quinton P.M, Zsembery Á. 2018. Bicarbonate inhibits bacterial growth and biofilm formation of prevalent cystic fibrosis pathogens. Front. Microbiol. 9, 2245. https://doi.org/10.3389/fmicb.2018.02245
Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. 2020. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 126 (10), 1456–1474. https://doi.org/10.1161/CIRCRESAHA.120.317015
Chigr F., Merzouki M., Najimi M. 2020. Autonomic brain centers and pathophysiology of COVID-19. ACS Chem. Neurosci. 11 (11), 1520–1522. https://doi.org/10.1021/acschemneuro.0c00265
Orsucci D., Ienco E.C., Nocita G., Napolitano A., Vista M. 2020. Neurological features of COVID-19 and their treatment. Drugs Context. 9, 2020-5-1. doi . eCollection 2020https://doi.org/10.7573/dic.2020-5-1
Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. 2008. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 82 (15), 7264–7275. https://doi.org/10.1128/JVI.00737-08
Li Y.C., Bai W.Z., Hashikawa T.J. 2020. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. Med. Virol. 92 (6), 552–555. https://doi.org/10.1002/jmv.25728
Tassorelli C., Mojoli F., Baldanti F., Bruno R., Benazzo M. 2020. COVID-19: What if the brain had a role in causing the deaths? Eur. J. Neurol.https://doi.org/10.1111/ene.14275
Dziewas R., Warnecke T., Zürcher P., Schefold J.C. 2020. Dysphagia in COVID-19 –multilevel damage to the swallowing network? Eur J Neurol. https://doi.org/10.1111/ene.14367
Montalvan V., Lee J., Bueso T., De Toledo J., Rivas K. 2020. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin. Neurol. Neurosurg. 194, 105921. https://doi.org/10.1016/j.clineuro.2020.105921
Serrano-Castro P.J., Estivill-Torrús G., Cabezudo-García P., Reyes-Bueno J.A., Ciano Petersen N., Aguilar-Castillo M.J., Suárez-Pérez J., Jiménez-Hernández M.D., Moya-Molina M.Á., Oliver-Martos B., Arrabal-Gómez C., de Fonseca F. R. 2020. Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: A delayed pandemic? Neurología (English Edition). 35 (4), 14 021–14 027. https://doi.org/10.1016/j.nrleng.2020.04.002
Iroegbu J.D., Ifenatuoha C.W., Ijomone O.M. 2020. Potential neurological impact of coronaviruses: Implications for the novel SARS-CoV-2. Neurol. Sci. 41 (6), 1329–1337. https://doi.org/10.1007/s10072-020-04469-4
Guzzi P.H., Mercatelli D., Ceraolo C., Giorgi F.M. 2020. Master regulator analysis of the SARS-CoV-2/ human interactome. J. Clin. Med. 9, 982–997. https://doi.org/10.3390/jcm9040982
Gulyaeva N.V., Bobkova N.V., Kolosova N.G., Samokhin A.N., Stepanichev M.Y., Stefanova N.A. 2017. Molecular and cellular mechanisms of sporadic Alzheimer’s disease: Studies on rodent models in vivo. Biochem. (Mosc). 82 (10), 1088–1102. https://doi.org/10.1134/S0006297917100029
Bobkova N.V., Lyabin D.N., Medvinskaya N.I., Samokhin A.N., Nekrasov P.V., Nesterova I.V., Aleksandrova I.Y., Tatarnikova O.G., Bobylev A.G., Vikhlyantsev I.M., Kukharsky M.S., Ustyugov A.A., Polyakov D.N., Eliseeva I.A., Kretov D.A., Guryanov S.G., Ovchinnikov L.P. 2015. The Y-Box binding protein 1 suppresses Alzheimer’s disease progression in two animal models. PLoS One. 10 (9). e0138867. https://doi.org/10.1371/journal.pone.0138867
Evgen’ev M.B., Krasnov G.S., Nesterova I.V., Garbuz D.G., Karpov V.L., Morozov A.V., Snezhkina A.V., Samokhin A.N., Sergeev A., Kulikov A.M., Bobkova N.V. 2017. Molecular mechanisms underlying neuroprotective effect of intranasal administration of human Hsp70 in mouse model of Alzheimer’s disease. J. Alz. Dis. 59 (4), 1415–1426. https://doi.org/10.3233/JAD-170398
Millet J.K., Whittaker G.R. 2015. Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis. Virus. Res. 202, 120–134. https://doi.org/10.1016/j.virusres.2014.11.021
Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada, T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. 2005. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 436 (7047), 112–116. https://doi.org/10.1038/nature03712
Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. 2005. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 11 (8), 875–879. https://doi.org/10.1038/nm1267
ethodical recommendations of the Ministry of Health of Russia “Prevention, diagnosis and treatment of new coronavirus infection COVID-19” (version 6 of 04/28/2020)
Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. 2020. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 55 (4), 105932. https://doi.org/10.1016/j.ijantimicag.2020.105932
Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 395 (10223), 497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Dayer M.R., Taleb-Gassabi S., Dayer M.S. 2017. Lopinavir; a potent drug against coronavirus infection: Insight from molecular docking study. Arch. Clin. Infect. Dis. 12 (4), 13823. https://doi.org/10.5812/archcid.13823
Lu H. 2020. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci. Trends. 14 (1), 69–71. https://doi.org/10.5582/bst.2020.01020
Hart B.J., Dyall J., Postnikova E., Zhou H., Kindrachuk J., Johnson R.F., Olinger G.G., Frieman M.B., Holbrook M.R., Jahrling P.B., Hensley L. 2014. Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J. Gen. Virol. 95 (Pt 3), 571–577. https://doi.org/10.1099/vir.0.061911-0
Barlow A., Landolf K.M., Barlow B., Yeung S.Y.A., Heavner J.J., Claassen C.W., Heavner M.S. 2020. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacother. 40 (5), 416–437. https://doi.org/10.1002/phar.2398
Wang Y., Jiang W., He Q., Wang C., Wang B., Zhou P., Dong N., Tong Q. 2020. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal. Transduct. Target. Ther. 5, 57. https://doi.org/10.1038/s41392-020-0158-2
Randomised Evaluation of COVID-19 Therapy (RECOVERY). 2020. Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. 2020. Available at: https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19. Accessed June 23, 2020.
Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., Rawling M., Savory E., Stebbing J. 2020. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 395 (10223), e30–e31. https://doi.org/10.1016/S0140-6736(20)30304-4
de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., Scott D., Cihlar T., Feldmann H. 2020. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA. 117 (12), 6771–6776. https://doi.org/10.1073/pnas.1922083117
Pardo J., Shukla A.M., Chamarthi G., Gupte A. 2020. The journey of remdesivir: From Ebola to COVID-19. Drugs. Context. 9, 2020-4-14. https://doi.org/10.7573/dic.2020-4-14
Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.M., Lim W.S., Makki S, Rooney K.D., Nguyen-Van-Tam J.S., Beck C.R. Convalescent plasma study group. 2015. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 211 (1), 80–90. https://doi.org/10.1093/infdis/jiu396
Iwasaki A., Pillai P.S. 2014. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 14 (5), 315–328. https://doi.org/10.1038/nri3665
Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth M.H., Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Taliaferro F., Mead B.E., Guo Z., Wang J.P., Gras D., Plaisant M., Ansari M., Angelidis I., Adler H., Sucre J.M.S., Taylor C.J., Lin B., Waghray A., Mitsialis V., Dwyer D.F., Buchheit K.M., Boyce J.A., Barrett N.A., Laidlaw T.M., Carroll S.L., Colonna L., Tkachev V., Peterson C.W., Yu A., Zheng H.B., Gideon H.P., Winchell C.G., Lin P.L., Bingle C.D., Snapper S.B., Kropski J.A., Theis F.J., Schiller H.B., Zaragosi L.E., Barbry P., Leslie A., Kiem H.P., Flynn J.L., Fortune S.M., Berger B., Finberg R.W., Kean L.S., Garber M., Schmidt A.G., Lingwood D., Shalek A.K., Ordovas-Montanes J. 2020. SARS-CoV-2 Receptor ACE2 Is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 181 (5), 1016–1035.e19. https://doi.org/10.1016/j.cell.2020.04.035
Fang L., Karakiulakis G., Roth M. 2020. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 8 (4), E21. https://doi.org/10.1016/S2213-2600(20)30116-8
Liu P.P., Blet A., Smyth D., Li H. 2020. The Science underlying COVID-19: Implications for the cardiovascular system. Circulation. 142 (1), 68–78. https://doi.org/10.1161/CIRCULATIONAHA.120.047549
Liu Y., Huang F., Xu J., Yang P., Qin Y., Cao M., Wang Z., Li X., Zhang S., Ye L., Lu J., Wei J., Xie T., Gao H., Xu K.-F., Wang F., Liu L., Jang C. 2020. Anti-hypertensive Angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. medRxiv. 2020.2003.2020.20039586. https://doi.org/10.1101/2020.03.20.20039586
Kreutz R., Algharably E.A.E., Azizi M., Dobrowolski P., Guzik T., Januszewicz A., Persu A., Prejbisz A., Riemer T.G., Wang J.G., Burnier M. 2020. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: Implications for COVID-19. Cardiovasc. Res. 116 (10), 1688–1699. https://doi.org/10.1093/cvr/cvaa097
Koka V., Huang X.R., Chung A.C., Wang W., Truong L.D., Lan H.Y. 2008. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am. J. Pathol. 172 (5), 1174–1183. https://doi.org/10.2353/ajpath.2008.070762
Patel V.B., Clarke N., Wang Z., Fan D., Parajuli N., Basu R., Putko B., Kassiri Z., Turner A.J., Oudit G.Y. 2014. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: A positive feedback mechanism in the RAS. J. Mol. Cell. Cardiol. 66, 167–176. https://doi.org/10.1016/j.yjmcc.2013.11.017
Scott A.J., O’Dea K.P., O’Callaghan D., Williams L., Dokpesi J.O., Tatton L., Handy J.M., Hogg P.J., Takata M. 2011. Reactive oxygen species and p38 mitogen-activated protein kinase mediate tumor necrosis factor α-converting enzyme (TACE/ADAM-17) activation in primary human monocytes. J. Biol. Chem. 286 (41), 35466–35476. https://doi.org/10.1074/jbc.M111.277434
Chappell M.C. 2016. Biochemical evaluation of the renin-angiotensin system: The good, bad, and absolute? Am. J. Physiol. Heart. Circ. Physiol. 310 (2), H137–H152. https://doi.org/10.1152/ajpheart.00618.2015
Santos R.A.S., Simoes e Silva A.C., Maric C., Silva D.M.R., Machado R.P., Buhr I., Heringer-Walther S., Pinheiro S.V.B., Teresa Lopes M., Bader M., Mendes E.P., Lemos V.S., Campagnole-Santos M.J., Schultheiss H.-P., Speth R., Walther T. 2003. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl. Acad. Sci. USA. 100 (14), 8258–8263.
Santos R.A., Ferreira A.J., Simões e Silva A.C. 2008. Recent advances in the angiotensin-converting enzyme 2–angiotensin(1–7)–Mas axis. Exp. Physiol. 93 (5), 519–527. https://doi.org/10.1113/expphysiol.2008.042002
Oudit G.Y., Kassiri Z., Patel M.P., Chappell M., Butany J., Backx P.H., Tsushima R.G., Scholey J.W., Khokha R., Penninger J.M. 2007. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc. Res. 75 (1), 29–39. https://doi.org/10.1016/j.cardiores.2007.04.007
Guzzi P.H., Mercatelli D., Ceraolo C., Giorgi F.M. 2020. Master regulator analysis of the SARS-CoV-2. Human. Interactome. J. Clin. Med. 9 (4), 982–997. https://doi.org/10.3390/jcm9040982
Verdecchia P., Cavallini C., Spanevello A., Angeli F. 2020. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 76, 14–20. https://doi.org/10.1016/j.ejim.2020.04.037
Magrone T., Magrone M., Jirillo E. 2020. Focus on receptors for coronaviruses with special reference to angiotensin-converting enzyme 2 as a potential drug target – a perspective. Endocr. Metab. Immune. Disord. Drug. Targets. 20 (6), 807–811. https://doi.org/10.2174/1871530320666200427112902
de Wit E., van Doremalen N., Falzarano D., Munster V.J. 2016. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 14 (8), 523–534. https://doi.org/10.1038/nrmicro.2016.81
Dong L., Hu S., Gao J. 2020. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov. & Therap. 14 (1), 58–60. doi.org/https://doi.org/10.5582/ddt.2020.01012
Prompetchara, E., Ketloy C., Palaga T. 2020. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian. Pac. J. Allergy. Immunol. 38 (1), 1–9. https://doi.org/10.12932/AP-200220-0772
Choy E.H., De Benedetti F., Takeuchi T., Hashizume M., John M.R., Kishimoto T. 2020. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 16 (6), 335–345. https://doi.org/10.1038/s41584-020-0419-z
Xie X.D., Chen J.Z., Wang X.X., Zhu J.H., Sun J., Tao M., Shang Y.P., Guo X.G. 2005. Cloning, expression and sequence analysis and tissue distribution of angiotensin-converting enzyme 2 (ACE2) gene in adult mice. Zhejiang Da Xue Xue Bao Yi Xue Ban. 34 (1), 48–54.
Tonnaer J.A., Engels G.M., Wiegant V.M., Burbach J.P., De Jong W., De Wied D. 1983. Proteolytic conversion of angiotensins in rat brain tissue. Eur. J. Biochem. 131 (2), 415–421. https://doi.org/10.1111/j.1432-1033.1983.tb07279.x
Santos R.A., Brosnihan K.B., Chappell M.C., Pesquero J., Chernicky C.L., Greene L.J., Ferrario C.M. 1988. Converting enzyme activity and angiotensin metabolism in the dog brainstem. Hypertension. 11 (2 Pt 2), 1153–1157. https://doi.org/10.1161/01.hyp.11.2_pt_2.i153
Ribeiro-Oliveira A., Nogueira A.I., Pereira R.M., Boas W.W.V., dos Santos R.A.S., e Silva A.C.S. 2008. The renin–angiotensin system and diabetes: An update. Vasc. Health. Risk. Manag. 4 (4), 787–803.
Rice G.I., Thomas D.A., Grant P.J., Turner A.J., Hooper N.M. 2004. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem. J. 383 (Pt 1), 45–51. https://doi.org/10.1042/BJ20040634
Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E, Acton S. 2000. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 87 (5), E1–E9. https://doi.org/10.1161/01.res.87.5.e1
Caruso-Neves C., Lara L.S., Rangel L.B., Grossi A.L., Lopes A.G. 2000. Angiotensin-(1–7) modulates the ouabain-insensitive Na+-ATPase activity from basolateral membrane of the proximal tubule. Biochim. Biophys. Acta, 1467 (1), 189–197. https://doi.org/10.1016/s0005-2736(00)00219-4
Lara L.S., Bica R.B.S., Sena S.L.F., Correa J.S., Marques-Fernandes M.F., Lopes A.G., Caruso-Neves C. 2002. Angiotensin-(1–7) reverts the stimulatory effect of angiotensin II on the proximal tubule Na+-ATPase activity via a A779-sensitive receptor. Regul. Pept. 103 (1), 17–22. https://doi.org/10.1016/s0167-0115(01)00322-6
Moreira de Macêdo S., Guimarães T.A., Feltenberger J.D., Sousa Santos S.H. 2014. The role of renin-angiotensin system modulation on treatment and prevention of liver diseases. Peptides. 62, 189–196. https://doi.org/10.1016/j.peptides.2014.10.005
Jiang T., Gao L., Guo J., Lu J., Wang Y., Zhang Y. 2012. Suppressing inflammation by inhibiting the NF-κB pathway contributes to the neuroprotective effect of angiotensin-(1–7) in rats with permanent cerebral ischaemia. Br. J. Pharmacol. 167 (7), 1520–1532. https://doi.org/10.1111/j.1476-5381.2012.02105.x
Jiang T., Gao L., Lu J. 2013. ACE2-Ang-(1-7)-Mas axis in brain: A potential target for prevention and treatment of ischemic stroke. Curr. Neuropharmacol. 11 (2), 209–217. https://doi.org/10.2174/1570159X11311020007
Wösten-van Asperen R.M., Lutter R., Specht P.A., Moll G.N., van Woensel,J.B., van der Loos C.M., van Goor H., Kamilic J., Florquin S., Bos A.P. 2011. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1–7) or an angiotensin II receptor antagonist. J. Pathol. 225 (4), 618–627. https://doi.org/10.1002/path.2987
Uhal B.D., Li X., Xue A., Gao X., Abdul-Hafez A. 2011. Regulation of alveolar epithelial cell survival by the ACE-2/angiotensin 1–7/Mas axis. Am. J. Physiol. Lung Cell. Mol. Physiol. 301 (3), L269–L274. https://doi.org/10.1152/ajplung.00222.2010
Jackson L.D., Eldahshan W., Fagan S.C., Ergul A. 2018. Within the brain: The renin angiotensin system. Int. J. Mol. Sci. 19 (3), 876. https://doi.org/10.3390/ijms19030876
Loseva E.V., Karnup S.V. 2004. Neurogenesis in the mature olfactory bulb and it’s possible functional destination. Usp. Fiziol. Nauk (Rus.). 35 (4), 11–18.
Kostenis E., Milligan G., Christopoulos A., Sanchez-Ferrer C.F., Heringer-Walther S., Sexton P.M., Gembardt F., Kellett E., Martini L., Vanderheyden P., Schultheiss H.P., Walther T. 2005. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 111 (14), 1806–1813. https://doi.org/10.1161/01.CIR.0000160867.23556.7D
da Silva Saldanha A.A., Rodrigues Prestes T.R., Lauar A.O., Finotti B.B., Simoes E., Silva A.C. 2017. Renin angiotensin system and cytokines in chronic kidney disease: Clinical and experimental evidence. Protein. Pept. Lett. 24 (9), 799–808. https://doi.org/10.2174/0929866524666170818160809
Dilek I., Henning R.H., van Gilst W.H., Roks A.J.M. 2008. Angiotensin-(1-7): Pharmacological properties and pharmacotherapeutic perspectives. Eur. J. Pharmacol. 585 (2–3), 303–312. https://doi.org/10.1016/j.ejphar.2008.02.090
Santos R.A., Campagnole-Santos M.J., Baracho N.C., Fontes M.A., Silva L.C., Neves L.A., Oliveira D.R., Caligiorne S.M., Rodrigues A.R., Gropen J.C. 1994. Characterization of a new angiotensin antagonist selective for angiotensin-(1-7): Evidence that the actions of angiotensin-(1-7) are mediated by specific angiotensin receptors. Brain Res. Bull. 35 (4), 293–298. https://doi.org/10.1016/0361-9230(94)90104-x
Wiemer G., Dobrucki L.W., Louka F.R. 2002. AVE 0991, a nonpeptide mimic of the effects of Angiotensin-(1–7) on the endothelium. Hypertension. 40 (6), 847–852. https://doi.org/10.1161/01.hyp.0000037979.53963.8f
Tirupula K.C., Desnoyer R., Speth R.C., Karnik S.S. 2014. Atypical signaling and functional desensitization response of MAS receptor to peptide ligands. PloS One. 9 (7), e103520. https://doi.org/10.1371/journal.pone.0103520
Hay M., Polt R., Heien M.L., Vanderah T.W., Largent-Milnes T.M., Rodgers K., Falk T., Bartlett M.J., Doyle K.P., Konhilas J.P. 2019. A Novel angiotensin-(1–7) glycosylated Mas receptor agonist for treating vascular cognitive impairment and inflammation-related memory dysfunction. J. Pharmacol. Exp. Ther. 369 (1), 9–25. https://doi.org/10.1124/jpet.118.254854
Savergnini S.Q., Beiman M., Lautner R.Q., de Paula-Carvalho V., Allahdadi K., Pessoa D.C., Costa-Fraga F.P., Fraga-Silva R.A., Cojocaru G., Cohen Y., Bader M., de Almeida A.P., Rotman G., Santos R.A.S. 2010. Vascular relaxation, antihypertensive effect, and cardioprotection of a novel peptide agonist of the MAS receptor. Hypertension. 56 (1), 112–120. https://doi.org/10.1161/HYPERTENSIONAHA.110.152942
Fandiño J., Vaz A. A., Toba L., Romaní-Pérez M., González-Matías L., Mallo F., Diz-Chaves Y. 2018. Liraglutide enhances the activity of the ACE-2/Ang(1–7)/Mas receptor pathway in lungs of male pups from food-restricted mothers and prevents the reduction of SP-A. Int. J.Endocrin. 2018, 6920620.https://doi.org/10.1155/2018/6920620
Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., Hall R., Poirier G., Ronco J.J., Tidswell M., Hardes K., Powley W.M., Wright T.J., Siederer S.K., Fairman D.A., Lipson D.A., Bayliffe A.I., Lazaar A.L. 2017. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care. 21, 234. https://doi.org/10.1186/s13054-017-1823-x
Paul M., Poyan M.A., Kreutz R. 2006. Physiology of local renin-angiotensin systems. Physiol. Rev. 86 (3), 747–803. https://doi.org/10.1152/physrev.00036.2005
Chapell M.C. 2016. Biochemical evaluation of the renin-angiotensin system: The good, bad, and absolute? Am. J. Physiol. Heart. Circ. Physiol. 310 (2), H137–H152. https://doi.org/10.1152/ajpheart.00618.2015
Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. 2005. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 436 (7047), 112–116. https://doi.org/10.1038/nature03712
Kobori H., Katsurada A., Miyata K., Ohashi N., Satou R., Saito T., Hagiwara Y., Miyashita K., Navar L.G. 2008. Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am. J. Physiol. Renal. Physiol. 294 (5), F1257–F1263. https://doi.org/10.1152/ajprenal.005882007
Campbell D.J., Duncan A.M., Kladis A., Harrap S.B. 1995. Angiotensin peptides in spontaneously hypertensive and normotensive Donryu rats. Hypertension. 25 (5), 928–934. https://doi.org/10.1161/01.hyp.25.5.928
Epelman S., Shrestha K., Troughton R.W., Francis G.S., Sen S., Klein A.L., Tang W.H. 2009. Soluble angiotensin-converting enzyme 2 in human heart failure: Relation with myocardial function and clinical outcomes. J. Card. Fail. 15 (7), 565–571. https://doi.org/10.1016/j.cardfail.2009.01.014
Luo H., Wang X., Chen C., Wang J., Zou X., Li C., Xu Z., Yang X., Shi W., Zeng C. 2015. Oxidative stress causes imbalance of renal renin angiotensin system (RAS) components and hypertension in obese Zucker rats. J. Am. Heart. Assoc. 4 (2), e001559. https://doi.org/10.1161/JAHA.114.001559
Nehme A., Zouein F.A., Zayeri Z.D., Zibara K. 2019. An update on the tissue renin angiotensin system and its role in physiology and pathology. J. Cardiovasc. Dev. Dis. 6 (2), 14. https://doi.org/10.3390/jcdd6020014
Engeli S., Negrel R., Sharma A.M. 2000. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension. 35 (6), 1270–1277. https://doi.org/10.1161/01.hyp.35.6.1270
Lee E.B., Fleischmann R., Hall S., Wilkinson B., Bradley J.D., Gruben D., Koncz T., Krishnaswami S., Wallenstein G.V., Zang C., Zwillich S.H., van Vollenhoven R.F. 2014. Tofacitinib versus methotrexate in rheumatoid arthritis. N. Engl. J. Med. 370 (25), 2377–2386. https://doi.org/10.1161/01.hyp.35.6.1270
Aazami F.S.H., Khoshmirsafa M., Kamali M., Mohsenzadegan M., Pornour M., Mansouri D. 2020. JAK inhibition as a new treatment strategy for patients with COVID-19. Int. Arch. Allergy. Immunol. 181 (6), 467–475. https://doi.org/10.1159/000508247
Li Y., Cao Y., Zeng Z., Liang M., Xue Y., Xi C., Zhou M., Jiang W. 2015. Angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas axis prevents lipopolysaccharide-induced apoptosis of pulmonary microvascular endothelial cells by inhibiting JNK/ NF–kB pathways. Sci. Rep. 5, 8209. https://doi.org/10.1038/srep08209
rendse L.B., Danser A.H.J., Poglitsch M., Touyz R.M., Burnett J.C., Llorens-Cortes C., Ehlers M.R. 2019. Therapeutic approaches targeting the renin-angiotensin system and associated peptides in hypertension and heart failure. Pharmacol. Rev. 71 (4), 539–570. 10.1124/pr.118.01712
Rojas A., Gonzalez I., Morales M.A. 2020. SARS-CoV-2-mediated inflammatory response in lungs: Should we look at RAGE? Inflamm. Res. 69 (7), 641–643. https://doi.org/10.1007/s00011-020-01353-x
Rojas A., Delgado-Lypez F., Gonzlez I., Perez-Castro R., Romero J., Rojas I. 2013. The receptor for advanced glycation end-products: A complex signaling scenario for a promiscuous receptor. Cell. Signal. 25 (3), 609–614. https://doi.org/10.1016/j.cellsig.2012.11.022
Bongarzone S., Savickas V., Luzi F., Gee A.D. 2017. Targeting the receptor for advanced glycation endproducts (RAGE): A medicinal chemistry perspective. J. Med. Chem. 60 (17), 7213−7232. https://doi.org/10.1021/acs.jmedchem.7b00058
Rojas A., Mercadal E., Figueroa H., Morales M.A. 2008. Advanced glycation and ROS: A link between diabetes and heart failure. Curr. Vasc. Pharmacol. 6 (1), 44–51. https://doi.org/10.2174/157016108783331312
Frank J.A., Briot R., Lee J.W., Ishizaka A., Uchida T., Matthay M.A. 2007. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am. J. Physiol. Lung. Cell. Mol. Physiol. 293 (1), L52–59. https://doi.org/10.1152/ajplung.00256.2006
Su X., Looney M.R., Gupta N., Matthay M.A. 2009. Receptor for advanced glycation end-products (RAGE) is an indicator of direct lung injury in models of experimental lung injury. Am. J. Physiol. Lung. Cell. Mol. Physiol. 297 (1), L1–5. https://doi.org/10.1152/ajplung.90546.2008
Shirasawa M., Fujiwara N., Hirabayashi S., Ohno H., Iida J., Makita K., Hata Y. 2004. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes. Cells. 9 (2), 165–174. https://doi.org/10.1111/j.1356-9597.2004.00712.x
Katsuoka F., Kawakami Y., Arai T., Imuta H., Fujiwara M., Kanma H., Yamashita K. 1997. Type II alveolar epithelial cells in lung express receptor for advanced glycation end products (RAGE) gene. Biochem. Biophys. Res. Commun. 238 (2), 512–516. https://doi.org/10.1006/bbrc.1997.7263
Helms M.N., Jain L., Self J.L., Eaton D.C. 2008. Redox regulation of epithelial sodium channels examined in alveolar type 1 and 2 cells patch-clamped in lung slice tissue. J. Biol. Chem. 283 (33), 22875–22883. https://doi.org/10.1074/jbc.M801363200
Downs C.A., Johnson N.M., Tsaprailis G., Helms M.N. 2018. RAGE-induced changes in the proteome of alveolar epithelial cells. J. Proteomics. 177, 11–20. https://doi.org/10.1016/j.jprot.2018.02.010
Faller S., Hausler F., Goeft A., von Itter M.N.A., Gyllenram V., Hoetzel A., Spassov S.G. 2018. Hydrogen sulfide limits neutrophil transmigration, inflammation, and oxidative burst in lipopolysaccharide-induced acute lung injury. Sci. Rep. 8 (1), 14676. https://doi.org/10.1038/s41598-018-33101
Zhang Z., Wang J., Xu G., Ding M., Liu F., Yuan L., Wang T., Xu J., Xie X., Deng B., Sun D., Lu W. 2019. Inhalation of sodium hydrosulfide (NaHS) alleviates NO and induced pulmonary function and hematological impairment in rats. Life. Sci. 232, 116650.https://doi.org/10.1016/j.lfs.2019.116650
Evgen’ev M.B., Frenkel A. 2020. Possible application of H2S-producing compounds in therapy of coronavirus (COVID-19) infection and pneumonia. Cell Stress and Chaperones. 25 (5), 713–715. https://doi.org/10.1007/s12192-020-01120-1
Pickering R.J., Tikellis C., Rosado C.J., Tsorotes D., Dimitropoulos A., Smith M, Huet O., Seeber R.M., Abhayawardana R., Johnstone E.K., Golledge J., Wang Y., Jandeleit-Dahm K.A., Cooper M.E., Pfleger K.D., Thomas M.C. 2019. Transactivation of RAGE mediates angiotensin-induced inflammation and atherogenesis. J. Clin. Invest. 129 (1), 406–421. https://doi.org/10.1172/JCI99987
Polverino F., Celli B.R., Owen C.A. 2018. COPD as an endothelial disorder: Endothelial injury linking lesions in the lungs and other organs? Pulm. Circ. 8 (1), 2045894018758528. https://doi.org/10.1177/2045894018758528
Prasad K. 2019. AGE-RAGE Stress in the pathophysiology of pulmonary hypertension and its treatment. Int. J. Angiol. 28 (2), 71–79. https://doi.org/10.1055/s-0039-1687818
Jeong J., Lee J., Lim J., Cho S., An S., Lee M., Yoon N., Seo M., Lim S., Park S. 2019. Soluble RAGE attenuates AngII-induced endothelial hyperpermeability by disrupting HMGB1-mediated crosstalk between AT1R and RAGE. Exp. Mol. Med. 51 (9), 1–15. https://doi.org/10.1038/s12276-019-0312-5
Pickering R.J., Tikellis C., Rosado C.J., Tsorotes D., Dimitropoulos A., Smith M., Huet O., Seeber R.M., Abhayawardana R., Johnstone E.K.M., Golledge J., Wang Y., Jandeleit-Dahm K.A., Cooper M.E., Pfleger K.D.G., Thomas M.C. 2019. Transactivation of RAGE mediates angiotensin-induced inflammation and atherogenesis. J. Clin. Invest. 129 (1). 406–421. https://doi.org/10.1172/JCI99987
Gilmore T.D., Garbati M.R. 2011. Inhibition of NF-kappaB signaling as a strategy in disease therapy. Curr. Top. Microbiol. Immunol. 349, 245–263. https://doi.org/10.1007/82_2010_105
Jia C., Zhang J., Chen H., Zhuge Y., Chen H., Qian F., Zhou K., Niu C., Wang F., Qiu H., Wang Z., Xiao J., Rong H., Chu M. 2019. Edothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation. Cell. Death. Dis. 10 (10), 778–794. https://doi.org/10.1038/s41419-019-2021-3
Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. 2020. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 20 (6), 363–374. https://doi.org/10.1038/s41577-020-0311-8
Prompetchara E., Ketloy C., Palaga T. 2020. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian. Pac. J. Allergy. Immunol. 38 (1), 1–9. https://doi.org/10.12932/AP-200220-0772
Funding
The author thanks Maria Dupont for the helpful discussion of peculiarities of the SARS-CoV-2 biology. The work was supported by the Russian Science Foundation (project no. 18-15-00392).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that she has no conflict of interest.
This article does not contain any studies involving animals or human participants performed by the author.
Additional information
Translated by A. Dunina-Barkovskaya
Rights and permissions
About this article
Cite this article
Bobkova, N.V. The Balance between Two Branches of RAS Can Protect from Severe COVID-19 Course. Biochem. Moscow Suppl. Ser. A 15, 36–51 (2021). https://doi.org/10.1134/S1990747821010037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747821010037