Abstract
The development of CAR-T specific therapy made a revolution in modern oncology. Despite the pronounced therapeutic effects, this novel approach displayed several crucial limitations caused by the complications in pharmacokinetics and pharmacodynamics controls. The presence of the several severe medical complications of CAR-T therapy initiated a set of attempts aimed to regulate their activity in vivo. We propose to apply the barnase-barstar system to control the cytotoxic antitumor activity of CAR-T cells. To menage the regulation targeting effect of the system we propose to use barstar-modified CAR-T cells together with barnase-based molecules. Barnase was fused with designed ankyrin repeat proteins (DARPins) specific to tumor antigens HER2 (human epidermal growth factor receptor 2) The application of the system demonstrates the pronounced regulatory effects of CAR-T targeting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
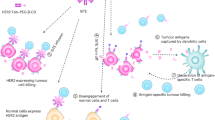
The development of anti-cancer therapeutics based on the genetically modified human blood cells displayed numerous positive results during the last decade [1, 2]. However, the lack of controlled pharmacokinetics and pharmacodynamics brings a substantial limitation in this approach showing a huge risk of the clinical side effects [3–6]. A numerous attempts to regulate tumor cytotoxicity caused by CAR-T cells were accomplished recently [7–9]. Some of them were concentrated on recruiting of FDA-approved drugs [10–14]. The specific protein-protein interaction may give a clue to solve the problem of an effective tuning of CAR-T cytotoxicity toward tumors. The RNase superfamily may be substantially inhibited by its cognate inhibitor barstar. The RNase-barstar complex is characterized by extremely tight binding with the extraordinary affinity (KD ∼10–14) [15, 16]. This makes sense to use the members of this complex for specific targeting of CAR-T cells and regulate cytotoxic antitumor effects. For this purpose, we designed two types of constructs, i.e. Barnase fused with previously designed ankyrin repeat proteins (DARPins) and barstar-modified CAR (BsCAR). We applied here the innovative approach for specific CAR-T targeting. We substituted the widely used targeting vectors based on the constructs from Ig superfamily by peptide scaffolds [17]. The applied DARPin is designed as a specific agent to tumor antigens HER2 (human epidermal growth factor receptor (Fig. 1).
The scheme of regulating the specific targeting of a T-cell modified with a universal BsCAR by the protein darpin-barnase (Da-Bn) targeting the HER2 of the tumor cell. The HER2 receptor has 4 extracellular subdomains: membrane-proximal-IV specific Da G3-Bn, membrane-distal I Da 9.29-Bn. Barnase interacts with high affinity with its inhibitor-barstar, which acts as a recognition domain in the CAR construct. GGGSGGGSGGGS and CH2-CH3 IgG4 are used as a hinge, CD28 TM/cyt = transmembrane and cytoplasmic domains from CD28, 4-1BB = cytoplasmic activation domain from CD137, CD3ζ = cytoplasmic activation domain from CD3ζ.
We are showing here that the DARPin-barnase (DARPin-Bn) protein is allowing to regulate specific targeting of tumor cells by T cells modified with universal BsCAR. We prepared a fusion proteins of anti-HER2 DARPin 9.29 (18) was used here as molecular “vector” directing BsCAR T cells against HER2+ tumor cells. DARPins 9.29 can bind with different domains of the HER2 receptor interacts with the membrane-distal subdomain I, with KD = 0.09 nM [19] and KD = 3.8 nM [18] respectively. We designed the scheme of the in vivo experiments with mice (BALB/c nude mice) (Fig. 2).
BsCAR T cells targeted by DARPin 9.29.-Bn suppress HER2-positive tumors in vivo. (a) Scheme of the experiment, where Balb/c Nu mice (females, 20–24 g) were intraperitoneally injected with 8 million SKOV-ip-Kat cells that form solid tumor nodes in the peritoneum. The presence of the red fluorescent protein TurboFP635 in the cytoplasm of these cells makes it possible to visualize the growth and spread of tumor nodes in mice using in vivo imaging technology using the IVIS SPectrum CT system (Perkin Elmer, USA). On day 2, 8 million, on day 4, 14 million BsCAR-T cells were intravenously injected into two groups of mice: the control group – BsCAR-T and the experimental group – BsCAR-T + darpin 9.29 – barnase. The therapy was performed by intraperitoneal administration of Da 9.29-Ba according to the scheme in the figure. Visualization of tumors was performed on the 5th and 10th days after the introduction of SKOV-ip-Kat cells. (b) IVIS images of animals treated with BsCAR-T cells alone or in combination with 9.29-barnase. The fluorescence of tumors of individual mice in each group is shown. (c) Tumor burden quantified as the mean fluorescence over 5 and 10 days. Data were analyzed using the Mann-Whitney test and presented as mean ± range. Statistical significance: *p < 0.05.
We may see from the data displayed on figure 1 that the proposed Barnase-Barstar system may specifically regulate antitumor cytotoxic effect of CAR T therapy. This system may control the efficacy of this therapy and may prevent the complications of CAR-T therapy and side effects.
Here we are showing the Modular CAR approach which is based on universal CARs that allows to separate two distinct process: (1) antigen recognition and (2) CAR T activation. This process might have perspectives shoving different redirecting agents. This may give rise the platform for CAR-T directed toward solid tumors.
METHODS
Obtaining BsCAR-T Cells
Obtaining a BsCAR lentiviral construct, producing CAR-T cells, and determining the level of BsCAR expression have been described previously [20]. The experiments used BsCAR-T cell products with a transduction level of 50% in the first cycle of administration and 40% in the second cycle of administration of BsCAR-T cells intravenously to immunodeficient BALB/c Nu mice.
In vivo Study
BALB/c Nu female mice, 8 weeks old, weighing 20–24 g, were taken for experiments. Animals were housed under specific pathogen-free conditions in the Pushchino Animal Breeding Facility IBCh RAS (Bioresource collection “Collection of laboratory rodents SPF category for basic, biomedical and pharmacological research”). Mice were intraperitoneally inoculated with 8 × 106 SKOV-ip-Kat cells, developed earlier [21], in 60% Matrigel in 100 µl of complete culture medium. On the second day, mice were randomized into 4 groups (n = 4). Animals from the 2nd and 4th groups were injected intravenously with 5 × 106 million on the 2nd day and 14 × 106 million on the 4th day of BsCAR-T cells. Four hours after injection of BsCAR-T cells, mice were intravenously injected intravenous of Da 9.29-barnase 100 μg in 50 μl PBS. The injection of Da 9.29-barnase was repeated on the 9th day of the experiment. The mice were weighed every other day and using the IVIS Spectrum In vivo Imaging System (PerkinElmer) on days 5 and 10.
Statistical Analysis
Statistical processing of the experimental results was performed using the Prism 9 software package (GraphPad Software). Tumor fluorescence value measurements were statistically processed using one-way analysis of variance (ANOVA) and analyzed using the Mann-Whitney test. Values are presented as mean ± range (n = 4). Statistical significance: *p < 0.05.
REFERENCES
Weber, E.W., Maus, M.V., and Mackall, C.L., The emerging landscape of immune cell therapies, Cell, 2020, vol. 181, pp. 46–62.
Stepanov, A.V. et al., Autocrine-based selection of ligands for personalized CAR-T therapy of lymphoma, Sci. Adv., 2018, vol. 4, p. eaau4580.
Turtle, C.J. et al., Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib, J. Clin. Oncol., 2017, vol. 35, pp. 3010–3020.
Mancikova, V. and Smida, M., Current state of CAR T-cell therapy in chronic lymphocytic leukemia, Int. J. Mol. Sci., 2021, vol. 22, p. 5536.
Kalinin, R.S. et al., Molecular approaches to safe and controlled engineered T-cell therapy, Acta Nat., 2018, vol. 10, pp. 16–23.
Schultz, L.M. et al., Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: a Pediatric Real-World Chimeric Antigen Receptor Consortium report, J. Clin. Oncol., 2022, vol. 40, pp. 945–955.
Davila, M.L. et al., Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia, Sci. Transl. Med., 2014, vol. 6, p. 224ra25.
Schuster, S.J. et al., Chimeric antigen receptor T cells in refractory B-cell lymphomas, N. Engl. J. Med., 2017, vol. 377, pp. 2545–2554.
Neelapu, S.S. et al., Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma, N. Engl. J. Med., 2017, vol. 377, pp. 2531–2544.
Urbanska, K. et al., A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor, Cancer Res., 2012, vol. 72, pp. 1844–1852.
Tamada, K. et al., Redirecting gene-modified T cells toward various cancer types using tagged antibodies, Clin. Cancer Res., 2012, vol. 18, pp. 6436–6445.
Ma, J.S.Y. et al., Versatile strategy for controlling the specificity and activity of engineered T cells, Proc. Natl. Acad. Sci. U. S. A., 2016, vol. 113, pp. E450–458.
Rodgers, D.T. et al., Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies, Proc. Natl. Acad. Sci. U. S. A., 2016, vol. 113, pp. E459–E468.
Cho, J.H., Collins, J.J., and Wong, W.W., Universal chimeric antigen receptors for multiplexed and logical control of T cell responses, Cell, 2018, vol. 173, pp. 1426–1438. e11.
Hartley, R.W., Barnase–barstar interaction, in Methods in Enzymology, Ribonucleases, part A, Nicholson, A.W., Ed., Academic Press, 2001, pp. 599–611.
Deyev, S.M., Waibel, R., Lebedenko, E.N., Schubiger, A.P., and Plückthun, A., Design of multivalent complexes using the barnase*barstar module, Nat. Biotechnol., 2003, vol. 21, pp. 1486–1492.
Tolmachev, V.M., Chernov, V.I., and Deyev, S.M., Targeted nuclear medicine. Seek and destroy, Russ. Chem. Rev., 2022, vol. 91, p. RCR5034.
Steiner, D., Forrer, P., and Plückthun, A., Efficient selection of DARPins with sub-nanomolar affinities using SRP phage display, J. Mol. Biol., 2008, vol. 382, pp. 1211–1227.
Zahnd, C. et al., A designed ankyrin repeat protein evolved to picomolar affinity to Her2, J. Mol. Biol., 2007, vol. 369, pp. 1015–1028.
Stepanov, A.V. et al., Switchable targeting of solid tumors by BsCAR T cells, Proc. Natl. Acad. Sci. U. S. A., 2022, vol. 119, p. e2210562119.
Zdobnova, T. et al., A novel far-red fluorescent xenograft model of ovarian carcinoma for preclinical evaluation of HER2-targeted immunotoxins, Oncotarget, 2015, vol. 6, pp. 30919–30928.
Funding
The work was supported by the Russian Science Foundation grant 17-74-30019 Structural and kinetic features of antigen presentation as a key to understanding the mechanisms of autoimmune pathology induction or lymphomogenesis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest.
The authors declare that they have no conflicts of interest.
Statement on the welfare of animals.
All procedures were approved by the IBCh RAS Institutional Animal Care and Use Committee (no. IACUC 729/20 of February 18, 2020).
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kalinin, R.S., Shipunova, V.O., Rubtsov, Y.P. et al. Barnase-barstar Specific Interaction Regulates Car-T Cells Cytotoxic Activity toward Malignancy. Dokl Biochem Biophys 508, 17–20 (2023). https://doi.org/10.1134/S1607672922700041
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1607672922700041