Abstract
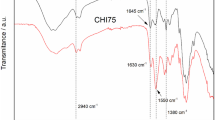
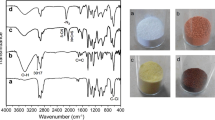
The influence of preparation conditions on the size characteristics and stability of particles of aqueous dispersions of positively and negatively charged polyelectrolyte complexes based on N-succinyl chitosan and poly-N,N-diallyl-N,N-dimethylammonium chloride was studied. An increase in the concentrations of the initial polymer solutions, irrespective of the order of their mixing and the charge of the complex, makes narrower the interval of the molar ratios of the initial polyelectrolytes at which the dispersions of particles of the complexes exhibit aggregation stability. The widest interval of molar ratios at which the dispersions are stable (0.20–0.55) is observed for systems of negatively charged complexes with the initial concentration of the components of 0.05%. In the presence of 0.1 М NaCl, the initial particle radius in both types of complexes decreases to 75–80 nm. Addition of more concentrated sodium chloride solutions, especially into systems based on positively charged complexes, leads to an increase in the initial particle radius to 90–700 nm. With time, the particle size increases, and variation of the component molar ratio does not noticeably affect the particle size of the complexes. The mean particle size of polyelectrolyte complexes in the region of relative sedimentation stability of disperse systems is 75–403 nm, which opens prospects for using these particles as drug carriers for targeted delivery.



Similar content being viewed by others
REFERENCES
Amjad, M.V., Farm. Farmakol.,, 2021, vol. 9, no. 1, pp. 4–16. https://doi.org/10.19163/2307-9266-2021-9-1-4-16
Gomzyak, V.I., Sedush, N.G., Puchkov, A.A., Polyakov, D.K., and Chvalun, S.N., Polym. Sci., Ser. B, 2021, vol. 63, no. 3, pp. 190–206. https://doi.org/10.1134/S1560090421030064
Zhao, D., Shuang Yu, Sun, B., Gao, S., Guo, S., and Zhao, K., Polymers (Basel), 2018, vol. 10, no. 4, pp. 462–477. PMID: 30966497. https://doi.org/10.3390/polym10040462
Izumrudov, V.A., Mussabayeva, B.Kh., Kassymova, Zh.S., Klivenko, A.N., and Orazzhanova, L.K., Russ. Сhem. Rev., 2019, vol. 88, no. 10, pp. 1046–1062. https://doi.org/10.1070/RCR4877.
Emmanuel, B.D., Abu-Thabit, N.Y., and Ngwuluka, N.C., Stim. Respons. Polym. Nanocarriers Drug Deliv. Appl., 2018, vol. 1, pp. 267–287. https://doi.org/10.1016/B978-0-08-101997-9.00014-X
Maiti, S., Jana, S., and Laha, B., in Design and Development of New Nanocarriers, William Andrew, 2018, pp. 223–256. https://doi.org/10.1016/B978-0-12-813627-0.00006-5
Bazunova, M.V., Sharafutdinova, L.A., Lazdin, R.Y., Chernova, V.V., Mixonov, D.N., and Zakharov, V.P., Appl. Biochem. Microbiol., 2018, vol. 54, no. 5, pp. 474–477. https://doi.org/10.1134/S0003683818050058
Skorik, Y.A., Kritchenkov, A.S., Moskalenko, Y.E., Golyshev, A.A., Raik, S.V., Whaley, A.K., Vasina, L.V., and Sonin, D.L., Carbohydr. Polym., 2017, vol. 166, pp. 166–172. https://doi.org/10.1016/j.carbpol.2017.02.097
Zhiryakova, M.V. and Izumrudov, V.A., Polym. Sci., Ser. A, 2008, vol. 50, no. 10, pp. 1057–1064. https://doi.org/10.1134/S0965545X08100064
Szafraniec-Szczęsny, J., Janik-Hazuka, M., Odrobińska, J., and Zapotoczny, S., Polymers, 2020, vol. 12, no. 9, pp. 1999–2024. https://doi.org/10.3390/polym12091999
Xian, J.L., J. Mol. Eng. Mater., 2017, vol. 5, no. 1, ID 1702001. https://doi.org/10.1142/S2251237317400019
Pharmacopoeia monograph FS.2.2.0020.15: Purified water, State Pharmacopoeia of the Russian Federation, Moscow, 2018, XIV ed.
Klenin, V.I., Termodinamika sistem s gibkotsepnymi polimerami (Thermodynamics of Systems with Flexible-Chain Polymers), Saratov: Saratovskii Univ., 1995.
Klenin, V.I., Shchegolev, S.Yu., and Lavrushin, V.I., Kharakteristicheskie funktsii svetorasseyaniya dispersnykh sistem (Characteristic Light Scattering Functions of Disperse Systems), Saratov: Saratovskii Univ., 1977.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated from Zhurnal Prikladnoi Khimii, No. 1, pp. 42–48, December, 2022 https://doi.org/10.31857/S0044461822010054
Rights and permissions
About this article
Cite this article
Bazunova, M.V., Mustakimov, R.A. & Bakirova, E.R. Conditions of Formation of Stable Polyelectrolyte Complexes Based on N-Succinyl Chitosan and Poly-N,N-diallyl-N,N-dimethylammonium Chloride. Russ J Appl Chem 95, 46–52 (2022). https://doi.org/10.1134/S1070427222010062
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427222010062