Abstract
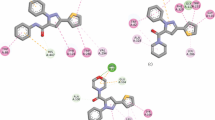
A series of substitute pyrazole compounds including azide, acetyl, triazole, morpholine, piperidine, and pyrrolidine moieties were synthesized and their structures were elucidated by NMR, HPLC and mass spectroscopy. The inhibition efficiencies of all novel compounds against acetylcholinesterase (AChE) and glutathione S-transferase (GST) enzymes were investigated. In vitro studies revealed that the inhibitory activities of substitute pyrazole compounds were determined with Ki values in the range of 0.11–0.49 µM for AChE, and 0.12–0.91 µM for GST, respectively. Furthermore, the molecular docking studies of the detailed interactions between the pyrazole compounds and AChE-GST enzymes were identified with bonding type, distance, hydrophobic bonds and hydrogen bonds. The binding energies of the AChE-pyrazole analogs’ complexes were found between –5.5 and –9.3 kcal/mol, and the binding energies of the GST-pyrazole analogs’ complexes were found between –5.9 and –9.2 kcal/mol.






Similar content being viewed by others
REFERENCES
Mathew, B., Parambi, D.G., Mathew, G.E., Uddin, M.S., Inasu, S.T., Kim, H., and Carradori, S., Arch. Pharm., 2019, vol. 352, no. 11, p. 1900177. https://doi.org/10.1002/ardp.201900177
Li, H., Su, Y.S., He, W., Zhang, J.B., Zhang, Q., Jing, X.H., and Zhan, L.B., FASEB J., 2022, vol. 36, no. 3, p. e22165. https://doi.org/10.1096/fj.202101529R
Danish, M., Jabeen, N., Raza, M.A., Mumtaz, M.W., and Tahir, M.N., Russ. J. Gen. Chem., 2021, vol. 91, p.294. https://doi.org/10.1134/S1070363221020183
Kalamida, D., Poulas, K., Avramopoulou, V., Fostieri, E., Lagoumintzis, G., Lazaridis, K., and Tzartos, S.J., FEBS J., 2007, vol. 274, p. 3799. https://doi.org/10.1111/j.1742-4658.2007.05935.x
Backos, D.S., Franklin, C.C., and Reigan, P., Biochem. Pharmacol., 2012, vol. 83, no. 8, p. 1005. https://doi.org/10.1016/j.bcp.2011.11.016
Jefferies, H., Coster, J., Khalil, A., Bot, J., McCauley, R.D., and Hall, J.C., ANZ J. Surg., 2003, vol. 73, no. 7, p. 517. https://doi.org/10.1046/j.1445-1433.2003.02682.x
Topuzyan, V.O., Hovhannisyan, A.A., Makichyan, A.T., and Hunanyan, L.S., Russ. J. Gen. Chem., 2022, vol. 92, no. 5, p. 819. https://doi.org/10.1134/S1070363222050115
Küçükgüzel, Ş.G. and Şenkardeş, S., Eur. J. Med. Chem., 2015, vol. 97, p. 786. https://doi.org/10.1016/j.ejmech.2014.11.059
Khan, M.F., Alam, M.M., Verma, G., Akhtar, W., Akhter, M., and Shaquiquzzaman, M., Eur. J. Med. Chem., 2016, vol. 120, p. 170. https://doi.org/10.1016/j.ejmech.2016.04.077
Wang, W., Liu, X.J., Lin, G.T., Wu, J.P., Xu, G., and Xu, D., Chem. Biodivers., 2022, vol. 19, no. 5, p. e202101032. https://doi.org/10.1002/cbdv.202101032
Kaur, G., Utreja, D., Jain, N., and Dhillon, N.K., Russ. J. Org. Chem., 2020, vol. 56, no. 1, p. 113. https://doi.org/10.1134/S1070428020010182
Ganguly, S. and Jacob, K.S., Mini-Rev. Med. Chem., 2017, vol. 17, no. 11, p. 959. https://doi.org/10.2174/1389557516666151120115302
Liu, L., Le, Y., Teng, M., Zhou, Z., Zhang, D., Zhao, C., and Cao, J., Dyes Pigm., 2018, vol. 151, p.1. https://doi.org/10.1016/j.dyepig.2017.12.005
Turkan, F., Cetin, A., Taslimi, P., Karaman, M., and Gulçin, I., Bioorg. Chem., 2019, vol. 86, p. 420. https://doi.org/10.1016/j.bioorg.2019.02.013
Trobe, M., and Burke, M.D., Angew. Chem. Int. Ed., 2018, vol. 57, no. 16, p. 4192. https://doi.org/10.1002/anie.201710482
Hura, N., Naaz, A., Prassanawar, S.S., Guchhait, S.K., and Panda, ACS Omega, 2018, vol. 3, no. 2, p. 1955.
Renyu, Q., Yuchao, L., Kandegama, W.M., Qiong, C., and Guangfu, Y., Mini-Rev. Med. Chem., 2018, vol. 18, no. 9, p. 781. https://doi.org/10.2174/1389557517666171101112850
Wiley, J.L., Burston, J.J., Leggett, D.C., Alekseeva, O.O., Razdan, R.K., Mahadevan, A., and Martin, B.R., Br. J. Pharmacol., 2005, vol. 145, no. 3, p. 293. https://doi.org/10.1038/sj.bjp.0706157
Yao, T.T., Xiao, D.X., Li, Z.S., Cheng, J.L., Fang, S.W., Du, Y.J., and Zhu, G.N., J. Agric. Food Chem., 2017, vol. 65, no. 26, p. 5397. https://doi.org/10.1021/acs.jafc.7b01251
Dubey, S., Prabitha, P., Bhardwaj, S., and Singh, E., Struct. Chem., 2019, vol. 30, no. 1, p. 263. https://doi.org/10.1007/s11224-018-1189-y
Kalaria, P.N., Karad, S.C., and Raval, D.K., Eur. J. Med. Chem., 2018, vol. 158, p. 917. https://doi.org/10.1016/j.ejmech.2018.08.040
Szekely, C.A., Thorne, J.E., Zandi, P.P., Ek, M., Messias, E., Breitner, J.C., and Goodman, S.N., Neuroepidemiology, 2004, vol. 23, p. 159. https://doi.org/10.1159/000078501
Onakpoya, I.J., Heneghan, C.J., and Aronson, J.K., Expert. Opin. Drug Saf., 2018, vol. 17, no. 1, p. 63. https://doi.org/10.1080/14740338.2018.1398232
Greish, K., Fateel, M., Abdelghany, S., Rachel, N., Alimoradi, H., Bakhiet, M., and Alsaie, A., J. Drug Target., 2018, vol. 26, no. 7, p. 610. https://doi.org/10.1080/1061186X.2017.1405427
Cluck, D., Lewis, P., Stayer, B., Spivey, J., and Moorman, J., Am. J. Health-Syst. Pharm., 2015, vol. 72, no. 24, p. 2135. https://doi.org/10.2146/ajhp150049
Boyer, E.W., Mejia, M., Woolf, A., and Shannon, M., Pediatrics, 2011, vol. 107, no. 1, p. 172. https://doi.org/10.1542/peds.107.1.172
Ansari, A., Ali, A., and Asif, M., New J. Chem., 2017, vol. 41, p. 16. https://doi.org/10.1039/C6NJ03181A
Faisal, M., Saeed, A., Hussain, S., Dar, P., and Larik, F.A., J. Chem. Sci., 2019, vol. 131, no. 8, p. 1. https://doi.org/10.1007/s12039-019-1646-1
Turkan, F., Cetin, A., Taslimi, P., Karaman, H.S., and Gulcin, I., Arch. Pharm., 2019, vol. 352, no. 10, p. 1800359. https://doi.org/10.1002/ardp.201800200
Anand. P., and Singh. B., Arch. Pharm. Res., 2013, vol. 36, no. 4, p. 375. https://doi.org/10.1007/s12272-013-0036-3
Daina, A., Michielin, O., and Zoete, V., Sci. Rep., 2017, vol. 7, no. 1, p. 13. https://doi.org/10.1038/srep42717
Hollenberg, P.F., Drug Metab. Rev., 2002, vol. 34, nos. 1–2, p. 35. https://doi.org/10.1081/DMR-120001387
Cetin, A., Bursal, E., and Türkan, F., Arab. J. Chem., 2021, vol. 14, no. 12, p. 103449. https://doi.org/10.1016/j.arabjc.2021.103449
Boy. S., Türkan, F., Beytur, M., Aras, A., Akyıldırım, O., Karaman, H.S., and Yüksek, H., Bioorg. Chem., 2021, vol. 107, p. 104524. https://doi.org/10.1016/j.bioorg.2020.104524
Adiguzel, R., Türkan, F., Yildiko, Ü., Aras, A., Evren, E., and Onkol, T., J. Mol. Struct., 2021, vol. 1231, p. 129943. https://doi.org/10.1016/j.molstruc.2021.129943
Habig, W.H., Pabst, M.J., and Jakoby, W.B., J. Biol. Chem., 1974, vol. 249, p. 7130. https://doi.org/10.1016/S0021-9258(19)42083-8
Mathew, N., Kalyanasundaram, M., and Balaraman, K., Expert. Opin. Ther. Pat., 2006, vol. 16, no. 4, p. 444. https://doi.org/10.1517/13543776.16.4.431
Cetin, A., Türkan, F., Bursal, E., and Murahari, M., Russ. J. Org. Chem., 2021, vol. 57, no. 4, p. 604. https://doi.org/10.1134/S107042802104014X
Ellman, G.L., Courtney, K.D., Andres, Jr.V., and Featherstone, R.M., Biochem. Pharm., 1961, vol. 7, p. 95. https://doi.org/10.1016/0006-2952(61)90145-9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Supplementary information
Rights and permissions
About this article
Cite this article
Cetin, A., Oguz, E. & Türkan, F. In Silico and In Vitro Analysis of Acetylcholinesteraseand Glutathione S-Transferase Enzymes of Substituted Pyrazoles. Russ J Gen Chem 92, 2415–2428 (2022). https://doi.org/10.1134/S1070363222110263
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222110263