Abstract
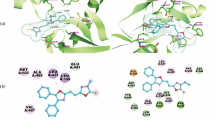
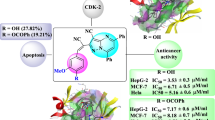
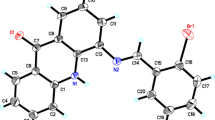
In order to produce new anti-cancer medications with various effects and minimum toxicity, a series of monastrol derivatives was synthesized by employing many potent anti-tumor aspects of ligustrazine derivatives as starting materials. The “combination principle” in drug discovery enlightened our thinking. Thus, the design and synthesis of monastrol analogues bearing ligustrazine moiety was achieved by the reaction of 2-thioxopyrimidine derivatives with 2-bromomethyl-3,5,6-trimethylpyrazine. Another analogue of monastrol was accomplished by the reaction of 2-thioxopyrimidine derivatives with chloroacetamide, which was then reacted with 2-bromomethyl-3,5,6-trimethylpyrazine to afford ligustrazine-containing monastrol derivatives. The antiproliferative activity was assessed for all monastrol derivatives including ligustrazine nucleus against three major types of human tumor cell lines, including breast MCF-7, colon HCT-116, and liver HepG-2 cancer cell lines. Studies on molecular docking were directed to the most potent cytotoxic compounds within the active site of kinesin Eg5 protein.












Similar content being viewed by others
REFERENCES
Cocco, M.T., Congiu, C., Lilliu, V., and Onnis, V., Bioorg. Med. Chem., 2006, vol. 14, no. 2, p. 366. https://doi.org/10.1016/j.bmc.2005.08.012
Elnaggar, D.H., Mohamed, A.M., Abdel Hafez, N.A., Azab, M.E., Elasasy, M.E., Awad, H.M., Farghaly, T.A., and Amr, A.E.-G.E., ACS Omega, 2022, vol. 7, no. 12, p. 10304. https://doi.org/10.1021/acsomega.1c06951
Mohamed, A.M., Abdelwahab, M., Elnaggar, D.H., Abde lHafez, N.A.; Mahmoud, S.F., El-Bayaa, M., El-kady, D.S., Omran, M.M., and El-Sayed, W.A., Egypt. J. Chem., 2022, vol. 65, no. 1, p. 1. https://doi.org/10.21608/ejchem.2021.84371.4127
Pedersen, O.S., Petersen, L., Brandt, M., Nielsen, C., and Pedersen, E.B., Monatshefte für Chemie/Chemical Monthly, 1999, vol. 130, no. 12, p. 1499. https://doi.org/10.1007/s007060050310
Fargualy, A.M., Habib, N.S., Ismail, K.A., Hassan, A.M., and Sarg, M.T., Eur. J. Med. Chem., 2013, vol. 66, p. 276. https://doi.org/10.1016/j.ejmech.2013.05.028
Hanna, M.M., Eur. J. Med. Chem., 2012, vol. 55, p. 12. https://doi.org/10.1016/j.ejmech.2012.06.048
Mohana, K.N., Kumar, B.N.P., and Mallesha, L., Drug Inνention Today, 2013, vol. 5, no. 3, p. 216. https://doi.org/10.1016/j.dit.2013.08.004
Fathalla, O., Awad, S., and Mohamed, M., Arch. Pharm. Res., 2005, vol. 28, no. 11, p. 1205. https://doi.org/10.1007/BF02978199
Shehta, W. and Abdel Hamid, A.M., Russ. J. Org. Chem., 2020, vol. 56, no. 5, p. 869. https://doi.org/10.1134/S1070428020050218
El-Mahdy, K.M., and Farouk, O., Russ. J. Org. Chem., 2020, vol. 56, no. 11, p. 2022. https://doi.org/10.1134/S1070428020110172
Lalpara, J.N., Vachhani, M.D., Hadiyal, S.D., Goswami, S., and Dubal, G.G., Russ. J. Org. Chem., 2021, vol. 57, no. 2, p. 241. https://doi.org/10.1134/S1070428021020159
Prachayasittikul, S., Worachartcheewan, A., Nantasenamat, C., Chinworrungsee, M., Sornsongkhram, N., Ruchirawat, S., and Prachayasittikul, V., Eur. J. Med. Chem., 2011, vol. 46, no. 2, p. 738. https://doi.org/10.1016/j.ejmech.2010.12.009
He, Y.-P., Long, J., Zhang, S.-S., Li, C., Lai, C.C., Zhang, C.-S., Li, D.-X., Zhang, D.-H., Wang, H., and Cai, Q.-Q., Bioorg. Med. Chem. Lett., 2011, vol. 21, no. 2, p. 694. https://doi.org/10.1016/j.bmcl.2010.12.003
Fathalla, O.A.E.-F.M., Ismail, M.A., Anwar, M.M., Abouzid, K.A., and Ramadan, A.A., Med. Chem. Res., 2013, vol. 22, no. 2, p. 659. https://doi.org/10.1007/s00044-012-0051-9
Leizerman, I., Avunie-Masala, R., Elkabets, M., Fich, A., and Gheber, L., Cell. Mol. Life Sci.CMLS, 2004, vol. 61, no. 16, p. 2060. https://doi.org/10.1007/s00018-004-4074-3
Haque, S.A., Hasaka, T.P., Brooks, A.D., Lobanov, P.V., and Baas, P.W., Cell Motil. Cytoskelet., 2004, vol. 58, no. 1, p. 10. https://doi.org/10.1002/cm.10176
Kumar, B.P., Sankar, G., and Baig, R.N., Eur. J. Med. Chem., 2009, vol. 44, no. 10, p. 4192. https://doi.org/10.1016/j.ejmech.2009.05.014
Kamal, A., Malik, M. S., Bajee, S., Azeeza, S., Faazil, S., Ramakrishna, S.,, Naidu, V., and Vishnuwardhan, M., Eur. J. Med. Chem., 2011, vol. 46, no. 8, p. 3274. https://doi.org/10.1016/j.ejmech.2011.04.048
Cheng, X., Liu, X., and Xu, W., Drugs Future, 2005, vol. 30, no. 10, p. 1059. https://doi.org/10.1358/dof.2005.030.10.927377
Pharmacopoeia, C., Commission Chinese Pharmacopoeia, Beijing: People’s Medical Publishing House, 2010, p. 1151.
Ding, M., Luo, S., Liu, H., Chen, P., and Liu, D., Chin. J. Chrom (Se pu), 2000, vol. 18, no. 1, p. 46
Li, Q.-Y., Gao, Y., Qiu, W., Zu, Y.-G.,Su, L., He, W.-N., and Deng, X.-Q., Lett. Drug Des. Discoνery, 2011, vol. 8, no. 8, p. 698. https://doi.org/10.2174/157018011796576006
Mohamed, A.M., Al-Qalawi, H.R., El-Sayed, W.A., Arafa, W.A., Alhumaimess, M.S., Hassan, A.K., Acta Poloniae Pharm. Drug Res., 2015, vol. 72, p. 307
Mohamed, A.M., El-Sayed, W.A., Alsharari, M.A., Al-Qalawi, H.R., Germoush, M.O., Arch. Pharm. Res., 2013, vol. 36, no. 9, p. 1055. https://doi.org/10.1007/s12272-013-0163-x
Amr, A.-G.E., Mohamed, A.M., Mohamed, S.F., AbdelHafez, N.A., and Hammam, A.E.-F.G., Bioorg. Med. Chem., 2006, vol. 14, no. 16, p. 5481. https://doi.org/10.1016/j.bmc.2006.04.045
Zaijun, Q.F.X. and Liang, Z., Patent Shenzhen Xiaxiwan Pharmaceutical Technology Co., Ltd., Junxia, CN107892673, 2018.
Xu, K., Wang, P., Xu, X., Han, Q., Wang, L., Li, Q., and Lei, H., Anhui Med. Pharm. J., 2013, vol. 17, p. 1467
Biginelli, C., Gazz. chim. Ital., 1893, vol. 23, no. 1, p. 360.
Kappe, C. O., Tetrahedron, 1993, vol. 49, no. 32, p. 6937. https://doi.org/10.1016/S0040-4020(01)87971-0
Mohamed, M.S., Awad, S.M., and Ahmed, N.M., Acta Pharm., 2011, vol. 61, no. 2, p. 171. https://doi.org/10.2478/v10007-011-0019-1
Ragab, F.A., Abou-Seri, S.M., Abdel-Aziz, S.A., Alfayomy, A.M., and Aboelmagd, M., Eur. J. Med. Chem., 2017, vol. 138, p. 140. https://doi.org/10.1016/j.ejmech.2017.06.026
Mohamed, M.S., Awad, S.M., Zohny, Y.M., and Mohamed, Z.M., Pharmacophore, 2012, vol. 3, no. 1, p. 62.
Shao, K., Zhang, X., Zhang, X., Xue, D., Ma, L., Zhang, Q., and Liu, H., Chin. J. Chem., 2014, vol. 32, no. 5, p. 443. https://doi.org/10.1002/cjoc.201400095
Hassan, A.S., Moustafa, G.O., Awad, H.M., Nossier, E.S., and Mady, M.F., ACS Omega, 2021, vol. 6, no. 18, p. 12361. https://doi.org/10.1021/acsomega.1c01604
Yan, Y., Sardana, V., Xu, B., Homnick, C., Halczenko, W., Buser, C.A., Schaber, M., Hartman, G.D., Huber, H.E., and Kuo, L.C., J. Mol. Biol., 2004, vol. 335, no. 2, p. 547. https://doi.org/10.1016/j.jmb.2003.10.074
Khattab, R.R., Alshamari, A.K., Hassan, A.A., Elganzory, H.H., El-Sayed, W.A., Awad, H.M., Nossier, E.S., and Hassan, N.A., J. Enzyme Inhib. Med. Chem., 2021, vol. 36, no. 1, p. 504. https://doi.org/10.1080/14756366.2020.1871335
Funding
The researchers extend their appreciation to the National Research Center (Dokki, Cairo, Egypt) for funding the work through the research group project no. 12010106.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Mohamed, A.M., Elnaggar, D.H., Elsayed, M.A. et al. Design, Docking Studies, and Anticancer Activity of Newly Synthesized Monastrol Analogues Bearing Ligustrazine Moiety. Russ J Gen Chem 92, 2400–2414 (2022). https://doi.org/10.1134/S1070363222110251
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222110251