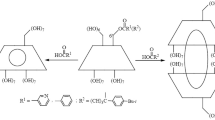
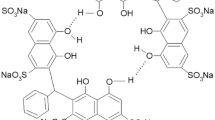
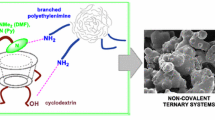
Abstract
We have summarized the works on the synthesis and application of dimeric (oligomeric) cyclodextrin derivatives. Due to the presence of two or more internal cyclodextrin cavities and their spatial proximity and other properties of these derivatives, they have an increased so-called cooperative effect with respect to the inclusion of numerous guests. It allows us to define these compounds as a new class of supramolecular structures. We have briefly analyzed the opportunities for their application in different fields of organic, analytical, and medicinal chemistry.

Similar content being viewed by others
REFERENCES
Len, Zh.-M., Supramolekulyarnaya khimiya: kontseptsii i perspektivy (Supramolecular Chemistry: Concepts and Prospects), Novosibirsk: Nauka, Sib. Predpr. Ross. Akad. Nauk, 1998.
Steed, J.W. and Atwood, J.L., Supramolecular Chemistry, Chichester: Wiley, 2000.
Dodziuk, H., Cyclodextrins and Their Complexes. Chemistry, Analytical Methods, Application, Wiley-VCH, Weinheim, 2006.
Chem. Rev. (special issue), 1998, vol. 98, no. 5.
Beilstein J. Org. Chem. (special issue), 2012, vol. 8.
Beilstein J. Org. Chem. (special issue), 2014, vol. 10.
Khan, A.R., Forgo, P., Stine, K.J., and D’Souza, V.T., Chem. Rev., 1998, vol. 98, no. 5, pp. 1977–1996.
Szejtli, J., Chem. Rev., 1998, vol. 98, no. 5, pp. 1743–1753.
Saenger, W., Jacob, J., Gessler, K., Steiner, T., Hoffman, D., Sanbe, H., Kozumi, K., Smith, S.M., and Tanaka, T., Chem. Rev., 1998, vol. 98, no. 5, pp. 1787–1802.
Croft, A.P. and Bartsch, R.A., Tetrahedron, 1983, vol. 39, no. 9, pp. 1417–1474.
Clazyrin, A.E., Kurochkina, G.I., Crachev, M.K., and Nifant’ev, E.E., Mendeleev Commun., 2001, no. 6, pp. 218–219.
Grachev, M.K., Glazyrin, A.E., Kurochkina, G.I., and Nifant’ev, E.E., Russ. J. Gen. Chem., 2004, vol. 74, pp. 808–809.
Glazyrin, A.E., Syrtsev, A.N., Kurochkina, G.I., Kononov, L.O., Crachev, M.K., and Nifant’ev, E.E., Russ. Chem. Bull., 2003, vol. 52, pp. 237–243.
Crachev, M.K., Russ. Chem. Rev., 2013, vol. 82, pp. 1034–1046.
de Miranda, J.C., Martins, T.E.A., Veiga, F., and Ferraz, H.G., Braz. J. Pharm. Sci., 2011, vol. 47, no. 4, pp. 665–681.
Harada, A., Furue, M., and Nozakura, S.I., Macromolecules, 1977, vol. 10, pp. 676–679.
Tabushi, I., Kuroda, Y., and Shimokawa, K., J. Am. Chem. Soc., 1979, vol. 101, pp. 1614–1615.
Harada, A., Furue, M., and Nozakura, S.I., Polymers, 1981, vol. 13, pp. 777–781.
Fujita, K., Ejima, S., and Imoto, T., Chem. Soc.,Chem. Commun., 1984, pp. 1277–1278.
Breslow, R., Greenspoon, N., Guo, T., and Zarzycki, R., J. Am. Chem. Soc., 1989, vol. 111, pp. 8296–8297.
Cho, E. and Jung, S., Molecules, 2015, vol. 20, pp. 19620–19646.
Liu, Y. and Chen, Y., Acc. Chem. Res., 2006, vol. 39, pp. 681–691.
Tilloy, S., Binkowski-Machut, C., Menuel, S., Bricout, H., and Monflier, E., Molecules, 2012, vol. 17, pp. 13062–13072.
Blaszkiewicz, C., Bricout, H., Leonard, E., Len, C., Landy, D., Cezard, C., Djedaϊni-Pilard, F., Monflier, E., and Tilloy, S., Chem. Commun., 2013, vol. 49, pp. 6989–6991.
Breslow, R., Belvedere, S., Gershell, L., and Leung, D., Pure Appl. Chem., 2000, vol. 72, no. 3, pp. 333–342.
Beilstein J. Org. Chem. (special issue), 2015, vol. 11.
Breslow, R. and Chung, S., J. Am. Chem. Soc., 1990, vol. 112, pp. 9659–9660.
Zhang, B. and Breslow, R., J. Am. Chem. Soc., 1993, vol. 115, pp. 9353–9354.
Zhang, B. and Breslow, R., J. Am. Chem. Soc., 1997, vol. 119, pp. 1676–1681.
Yan, J. and Breslow, R., Tetrahedron Lett., 2000, vol. 41, pp. 2059–2062.
Breslow, R., Yang, Z., Ching, R., Trojandt, G., and Odobel, F., J. Am. Chem. Soc., 1998, vol. 120, pp. 3536–3537.
Zutschi, R., Brickner, M., and Chmielewski, J., Curr. Opin. Chem. Biol., 1998, vol. 2, pp. 62–66.
Chmielewski, J., Biopolymers, 1998, vol. 47, pp. 277–283.
Zutshi, R., Shultz, M.D., Ulysse, L., Lutgring, R., Bishop, P., Schweitzer, B., Vogel, K., Franciskovich, J., Wilson, M., and Chmielewski, J., Synlett, 1998, vol. 10, pp. 1040–1044.
Zutshi, R., Franciskovich, M., Shultz, M., Schweitzer, B., Bishop, M., Wilson, M., and Chmielewski, J., J. Am. Chem. Soc., 1997, vol. 119, pp. 4841–4845.
Daugherty, D.L. and Gellman, S.H., J. Am. Chem. Soc., 1999, vol. 121, pp. 4325–4333.
Park, H.S., Lin, Q., and Hamilton, A.D., J. Am. Chem. Soc., 1999, vol. 121, pp. 8–13.
Lin, Q., Park, H.S., Humaro, Y., Lee, C.S., and Hamilton, A.D., Biopolymers, 1998, vol. 47, pp. 285–297.
Clackson, T., Ultsch, M.H., Wells, J.A., and de Vos, A.M., J. Mol. Biol., 1998, vol. 277, pp. 1111–1128.
Ruebner, A., Yang, Z., Leung, D., and Breslow, R., Proc. Natl. Acad. Sci. U. S. A., 1999, vol. 96, pp. 14692–14693.
Liy, Y., Chen, G.-S., Li, L., Zhang, H.-Y., Cao, D.-X., and Yuan, Y.-J., J. Med. Chem., 2003, vol. 46, pp. 4634–4637.
Aykaҫ, A., Martos-Maldonado, M.C., Casas-Solvas, J.M., Garsiá-Fuentes, L., and Vargas-Berenguel, A., J. Drug Del. Sci. Tech., 2012, vol. 22, pp. 270–272.
Kritskiy, I., Kumeev, R., Volkova, T., Shipilov, D., Kutysheva, N., Grachev, M., and Terekhova, I., New J. Chem., 2018, vol. 42, pp. 14559–14567.
Grachev, M.K., Malenkovskaya, M.A., and Vasyanina, L.K., J. Incl. Phenom. Macrocycl. Chem., 2015, vol. 83, nos. 3–4, pp. 209–214.
Malenkovskaya, M.A., Vasyanina, L.K., and Grachev, M.K., Russ. J. Gen. Chem., 2015, vol. 85, pp. 1161–1165.
Breslow, R., Zhang, X., and Huang, Y., J. Am. Chem. Soc., 1997, vol. 119, pp. 4535–4536.
Breslow, R., Gabriele, B., and Yang, J., Tetrahedron Lett., 1998, vol. 39, pp. 2887–2890.
Breslow, R. and Zhang, B., J. Am. Chem. Soc., 1996, vol. 118, no. 35, pp. 8495–8496.
Leung, D.K., Atkins, J.H., and Breslow, R., Tetrahedron Lett., 2001, vol. 42, no. 36, pp. 6255–6258.
Bistri, O., Lecourt, T., Mallet, J.-M., Sollogoub, M., and Sinaӱ, P., Chem. Biodiversity, 2004, vol. 1, no. 1, pp. 129–137.
Lecourt, T., Mallet, J.-M., and Sinaӱ, P., Tetrahedron Lett., 2002, vol. 43, pp. 5533–5536.
Chen, Y., Li, L., and Liu, Y., Chem. J. Chin. Univ., 2002, vol. 23, no. 6, pp. 1091–1093.
Zheng, X.Q., Wang, Y.H., Guo, Q.X., Yang, L., and Liu, Y.C., Chin. Chem. Lett., 2003, vol. 14, no. 8, pp. 848–851.
Song, L.X., Chin. Chem. Lett., 2002, vol. 13, no. 10, pp. 1005–1006.
Zhao, Y., Liu, X.Q., Wang, L.-Q., Zhu, H.-Y., Huang, R., Wang, Y.-F., and Yang, Z.-M., J. Phys. Org. Chem., 2008, vol. 21, no. 6, pp. 440–448.
Pham, D.-T., Ngo, H.T., Lincoln, S.F., May, B.L., and Easton, C.J., Tetrahedron, 2010, vol. 66, pp. 2895–2898.
Takashima, Y., Yuting, Y., Otsubo, M., Yamaguchi, H., and Harada, A., Beilstein J. Org. Chem., 2012, vol. 8, pp. 1594–1600.
Malenkovskya, M.A., Levina, I.I., and Grachev, M.K., J. Org. Chem., 2014, vol. 50, pp. 1211–1215.
Shipilov, D.A., Kurochkina, G.I., Akhlebinin, A.K., Kutyasheva, N.V., and Grachev, M.K., Macroheterocycles, 2019, vol. 12, no. 1, pp. 94–97.
Tripodo, G., Wischke, C., Neffe, A.T., and Lendlein, A., Carbohydr. Res., 2013, vol. 381, pp. 59–63.
Kurlemann, M. and Kavoo, B.J., Beilstein J. Org. Chem., 2014, vol. 10, pp. 2428–2440.
Hamon, F., Blaszkiewicz, C., Monflier, E., and Djedaim-Pilard, F., Beilstein J. Org. Chem., 2014, vol. 10, pp. 2874–2885.
Kuad, P., Miyawaki, A., Takashima, Y., Yamaguchi, H., and Harada, A., J. Am. Chem. Soc., 2007, vol. 129, no. 42, pp. 12630–12631.
Ohga, K., Takashima, Y., Takashima, Y., Kawaguchi, Y., Yamaguchi, H., and Harada, A., Macromolecules, 2005, vol. 38, no. 14, pp. 5897–5904.
Städe, L.W., Nielsen, T.T., Duroux, L., Wimmer, R., Shimizu, K., and Larsen, K.L., Beilstein J. Org. Chem., 2015, vol. 11, pp. 514–523.
Trotta, F., Zanetti, M., and Cavelli, R., Beilstein J. Org. Chem., 2012, vol. 8, pp. 2091–2099.
Maurer, M., Hapiot, F., Monflier, E., and Menuel, S., Tetrahedron, 2008, vol. 64, pp. 7159–7163.
Manuel, S., Azaroul, N., Landy, D., Six, N., Hapiot, F., and Monflier, E., Chem.-Eur. J., 2011, vol. 17, pp. 3949–3955.
Voskuhl, J., Schaepc, K., and Ravoo, B.J., Int. J. Mol. Sci., 2011, vol. 12, pp. 4637–4646.
Terao, J., Konoshima, Y., Matono, A., Masai, H., Fujihara, T., and Tsuji, Y., Beilstein J. Org. Chem., 2014, vol. 10, pp. 2800–2808.
Edwards, W.B., Reichert, D.E., d’Avignon, D.A., and Welch, M.J., Chem. Commun., 2001, pp. 1312–1313.
Casas-Solvas, J.M., Quesad-Soriano, I., Carreno-Gazquez, D., Gimenez-Martinez, J.J., Garcia-Fuentes, L., and Vargas-Berenguel, Langmuir, 2011, vol. 27, pp. 9729–9737.
Venema, F., Baselier, C.M., van Dienst, E., Ruel, B.H.M., Feiters, M.C., Endersen, J.F.J., Reinhoudt, D.N., and Nolte, R.J.M., Tetrahedron Lett., 1994, vol. 35, no. 11, pp. 1773–1776.
Yan, J. and Breslow, R., Tetrahedron Lett., 2000, vol. 41, no. 13, pp. 2059–2062.
von Piekartz, I., Grote, GanseyM.H.B., Venema, F., Feiters, M.C., Nolte, R.J.M., Engbersen, J.F.J., and Reinhoudt, D.N., Org. Chem., 1995, vol. 60, pp. 6537–6545.
Jiang, T., Sukumaran, D.K., Soni, S.-D., and Lawrence, D.S., Org. Chem., 1994, vol. 59, no. 18, pp. 5149–5155.
Chiu, S.-H., Myles, D.C., Garrell, R.L., and Stoddart, J.F., Org. Chem., 2000, vol. 65, no. 9, pp. 2792–2796.
De Jong, M.R., Engbersen, J.F.J., Huskens, J., and Reinhoudt, D.N., Chem.-Eur. J., 2000, vol. 6, pp. 4034–4040.
Jia, S.-Y., Hao, Y.-Q., Li, L.-N., Chen, K., Wu, Y., Liu, J., Wu, L., and Ding, Y.-H., Chem. Lett., 2005, vol. 34, no. 9, pp. 1248–1249.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain studies that use humans and animals as objects of research.
Conflict of Interests
The authors state that there is no conflict of interest.
Additional information
Translated by A. Levina
Corresponding author: phone: +7 (495) 682-02-45; e-mail: mkgrachev@yandex.ru.
Rights and permissions
About this article
Cite this article
Grachev, M.K., Terekhova, I.V., Shipilov, D.A. et al. Dimeric (Oligomeric) Derivatives of Cyclodextrins as a New Class of Supramolecular Systems: Their Synthesis and Inclusion Complexes. Russ J Bioorg Chem 46, 14–31 (2020). https://doi.org/10.1134/S1068162020010021
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162020010021