Abstract
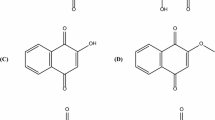
Coumarins are a well-known group of natural products distributed in the plant kingdom especially in the family Apiaceae with various biological activities. Isoarnottinin 4′-glucoside is a simple glycosylated coumarin found previously in a few genera of Apiaceae, and its biological activities have not been previously described in details. In the present paper, the compound was isolated from Prangos uloptera (Apiaceae) leaves using HPLC techniques. Antimicrobial, phytotoxic and cytotoxic activities of the compound were evaluated by disk diffusion, lettuce assay and MTT method. Our results indicated that the compound has high antibacterial effect against Erwinia carotovora, a common plant pathogen with MIC value of 100 μg/ml. The compound also exhibited significant phytotoxic activity against lettuce and modest cytotoxic activity against HeLa cell line with IC50 of 0.84 mg/ml. It could be concluded that isoarnottinin 4′-glucoside may play phytoalexin or allelopathic role for plant and may be a candidate for an antibacterial agent or a bioherbicide.
Similar content being viewed by others
References
Heldt, H.W., Plant Biochemistry, 3rd ed., London: Elsevier, 2005.
Ikan, P., Selected Topics in the Chemistry of Natural Products, Singapore: World Sci. Publ., 2008.
Rahman, A.U., Studies in Natural Product Chemistry, vol 24, Amsterdam: Elsevier, 2000.
Elgamal, M.H.A., Shalchy, N.M.M., Duddeck, H., and Hiegemann, M., Phytochemistry, 1993, vol. 34, pp. 819–823.
Buckingham, J., Dictionary of Natural Products on CD-ROM, V.13:1, Boca Raton: Chapman Hall/CRC Press, 2005.
Razavi, S.M., Nazemiyeh, H., Delazar, A., Hajiboland, R., Kumarssamy, Y., Nahar, L., and Sarker, S.D., Brazil. J. Pharmacognosy, 2008, vol. 18, pp. 1–5.
Razavi, S.M., Nazemiyeh, H., Delazar, A., Hajiboland, R., Mukhlesur Rahman, M., Gibbona, S., Nahar, L., and Sarker, S.D., Phytochem. Letts., 2008, vol. 1, pp. 159–162.
Strange, R.N., Introduction to Plant Pathology, West Sussex: Wiley, 2003.
Lyon, G.D. and McGill, F.M., Potato Res., 1988, vol. 31, pp. 461–467.
Montenegro, G., Portaluppi, M.C., Salas, F.A., and Diaz, M.F., Biol. Res., 2009, vol. 42, pp. 233–237.
Naylov, R.E.L., Weed Management Handbook, 9th ed., Oxford: Blackwell, 2002.
Kostova, I., Curr. Med. Chem., 2005, vol. 5, pp. 29–46.
Razavi, S.M., Zahri, S., Zarrini, G., Nazemiyeh, H., Mohammadi, S., and Abolghasemi, M.A., Natur. Products Res., 2009, vol. 23, pp. 1522–1527.
Razavi, S.M., Imanzadeh, G.H., and Davari, M., Euras. J. Biosci., 2010, vol. 4, pp. 17–22.
Razavi, S.M., Zahri, S., Zarrini, G., Nazemiyeh, H., and Mohammadi, S., Russ. J. Bioorg. Chem., 2009, vol. 35, pp. 376–378.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Razavi, S.M., Zarrini, G. & Rad, F.G. Isoarnottinin 4′-glucoside, a glycosylated coumarin from Prangos uloptera, with biological activity. Russ J Bioorg Chem 37, 240–243 (2011). https://doi.org/10.1134/S1068162011020130
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162011020130