Abstract
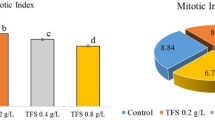
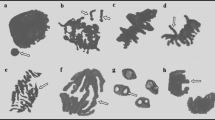
The toxicity profile of coronatine (COR), a toxin produced by Pseudomonas bacteria using the Allium cepa test plant was determined with the help physiological analyzes including fresh weight, root number, root length and germination percentage; cytogenetic analyzes including chromosome aberration (CA), micronucleus frequency (MN), and mitotic index (MI); biochemical analyzes including superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA) and proline (PR) accumulation; and microscopic analyzes including changes in root anatomical structure. A. cepa bulbs were divided into four groups as one control (C) and three application. Group C bulbs were kept in cuvettes containing tap water for 168 hours (7 days), while treatment group bulbs were kept in cuvettes containing 1, 5, and 10 µM COR solutions. COR administration caused a decrease in all physiological parameters examined, a rise in the CA and MN frequency, also a diminish in MI compared to C group. COR promoted CAs such as irregular mitosis, nuclear peak, exposure of chromosome scaffold, chromosome losses, unequal seperation of chromosome, vagrant chromosomes and chromatid bridges. In addition, the mentioned application caused a dosege-bound enhancement in free PR, CAT, SOD and MDA contents according to C group. Moreover, 10 µM COR, the highest application dose, caused quite significant damages such as epidermis cell deformations, micronucleus in epidermis/cortex, accumulation of various chemicals in the cortex layer, thickening of the cortex cell wall, flattened cell nuclei, necrosis and unclear transmission tissue in root anatomical structure of the bulbs. In summary, it was concluded that COR is a chemical with inhibitive impacts and the Allium cepa testing is a utility bioindicator for following these impacts.







Similar content being viewed by others
REFERENCES
Abbas, T., Nadeem, M.A., Tanveer, A., and Chauhan, B.S., Can hormesis of plant released phytotoxins be used to boost and sustain crop production?, Crop Protect., 2017, vol. 93, pp. 69–76.
Akgunduz, M.C., Cavusoglu, K., and Yalcin, E., The potential risk assessment of phenoxyethanol with a versatile model system, Sci. Rep., 2020, vol. 10, pp. 1209–1218.
Andrade, L.F., Davide, L.C., and Gedraite, L.S., The effect of cyanide compounds, fluorides, aluminum, and inorganic oxides present in spent pot liner on germination and root tip cells of Lactuca sativa, Ecotoxicol. Environ. Saf., 2010, vol. 73, pp. 626–631.
Baker, A.J.M., Accumulators and excluders-strategies in the response of plants to heavy metals, J. Plant Nutr., 1981, vol. 3, pp. 643–654.
Bates, L.S., Waldren, R.P., and Teare, I.D., Rapid determination of free proline for water stress studies, Plant Soil., 1973, vol. 39, pp. 205–207.
Beauchamp, C. and Fridovich, I., Superoxide dismutase: improved assays and an assay applicable to acrylamide gels, Anal. Biochem., 1971, vol. 44, pp. 276–287.
Beers, R.F. and Sizer, I.W., Colorimetric method for estimation of catalase, J. Biol. Chem., 1952, vol. 195, pp. 133–139.
Boughalleb, F., Abdellaoui, R., Mahmoudi, M., and Bakhshandeh, E., Changes in phenolic profile, soluble sugar, proline, and antioxidant enzyme activities of Polygonum equisetiforme in response to salinity, Turk. J. Bot., 2020, vol. 44, pp. 25–35.
Brooks, D.M., Bender, C.L., and Kunkel, B.N., The Pseudomonas syringae phytotoxin coronatine promotes virulence by overcoming salicylic acid-dependent defenses in Arabidopsis thaliana, Mol. Plant Pathol., 2005, vol. 6, pp. 629–639.
Bucheli, T.D., Phytotoxins: Environmental Micropollutants of Concern?, ACS Publications, 2014.
Celik, O. and Atak, C., The effect of salt stress on antioxidative enzymes and proline content of two Turkish tobacco varieties, Turk. J. Biol., 2012, vol. 36, pp. 339–356.
Ceylan, H.A., Turkan, I., and Sekmen, A.H., Effect of coronatine on antioxidant enzyme response of chickpea roots to combination of PEG-induced osmotic stress and heat stress, J. Plant Growth Regul., 2013, vol. 32, pp. 72–82.
Chaparro, T.R., Botta, C.M., and Pires, E.C., Biodegradability and toxicity assessment of bleach plant effluents treated anaerobically, Water Sci. Technol., 2010, vol. 62, pp. 1312–1319.
Chen, H., Singh, H., Bhardwaj, N., Bhardwaj, S.K., Khatri, M., Kim, K.H., and Peng, W., An exploration on the toxicity mechanisms of phytotoxins and their potential utilities, Crit. Rev. Environ. Sci. Technol., 2020, vol. 52, no. 3, pp. 395–435.
Dinakar, C., Abhaypratap, V., Yearla, S.R., Raghavendra, A.S., and Padmasree, K., Importance of ROS and antioxidant system during the beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation, Planta, 2010, vol. 231, pp. 461–474.
El-Ghamery, A.A., El-Kholy, M.A., and Abou El-Yousser, M.A., Evaluation of cytological effects of Zn2+ in relation to germination and root growth of Nigella sativa L. and Triticum aestivum L., Mutat. Res., 2003, vol. 537, pp. 29–41.
Fedina, I.S. and Benderliev, K.M., Response of Scendesmus incrassatulus to salt stress as affected by methyl jasmonate, Biol. Plant., 2000, vol. 43, pp. 625–627.
Fernandes, T.C.C., Mazzeo, D.E.C., and Marin Morales, M.A., Mechanism of micronuclei formation in polyploidizated cells of A. cepa exposed to trifluralin herbicide, Pest. Biochem. Physiol., 2007, vol. 88, pp. 252–259.
Geng, X.Q., Jin, L., Shimada, M., Kim, M.G., and Mackey, D., The phytotoxin coronatine is a multi functional component of the virulence armament of Pseudomonas syringae, Planta, 2014, vol. 240, pp. 1149–1165.
Grant, W.F., Higher plant assays for the detection of chromosomal aberrations and gene mutations-a brief historical back ground on their use for screening and monitoring environmental chemicals, Mutat. Res., 1999, vol. 426, pp. 107–112.
Greulich, F., Yoshihara, T., and Ichihara, A., Coronatine, a bacterial phytotoxin, acts as a stereospecific analog of jasmonate type signals in tomato cells and potato tissues, J. Plant Physiol., 1995, vol. 147, pp. 359–366.
Harashima, H. and Schnittger, A., The integration of cell division, growth and differentiation, Curr. Opin. Plant Biol., 2010, vol. 13, pp. 66–74.
Hao, L., Wang, Y.Q., Zhang, J., Xie, Y., Zhang, M.C., Duan, L.S., and Li, Z.H., Coronatine enhances drought tolerance via improving antioxidative capacity to maintaining higher photosynthetic performance in soybean, Plant Sci., 2013, vol. 210, pp. 1–9.
Janero, D.R., Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury, Free Radic. Biol. Med., 1990, vol. 9, pp. 515–540.
Kalcheva, V.P., Dragoeva, A.P., Kalchev, K.N., and Enchev, D.D., Cytotoxic and genotoxic effects of Br-containing oxaphosphole on Allium cepa L. root tip cells and mouse bone marrow cells, Genet. Mol. Biol., 2009, vol. 32, pp. 389–393.
Kenyon, J.S. and Turner, J.G., Physiological changes in Nicotiana tobacum leaves during development of chlorosis caused by coronatine, Physiol. Mol. Plant Pathol., 1990, vol. 37, pp. 463–477.
Kenyon, J.S. and Turner, J.G., The stimulation of ethylene synthesis in Nicotiana tabacum leaves by the phytotoxin coronatine, Plant Physiol., 1992, vol. 100, no. 1, pp. 219–224.
Lauchli, R. and Boland, W., Indanoyl amino acid conjugates: tunable elicitors of plant secondary metabolism, Chem. Rec., 2003, vol. 3, pp. 12–21.
Leme, D.M. and Marin-Morales, M.A., Allium cepa test in environmental monitoring: a review on its application, Mutat. Res., 2009, vol. 82, pp. 71–81.
Liu, H., Liao, B., and Lu, S.Q., Toxicity of surfactant, acid rain and Cd2+ combined pollution to the nucleus of Vicia faba root tip cells, Chin. J. Appl. Ecol., 2004, vol. 15, pp. 493–496.
Li, Y., Huang, G., Guo, Y., Zhou, Y., and Duan, L., Coronatine enhances stalk bending resistance of maize, thickens the cell wall and decreases the area of the vascular bundles, Agronomy., 2020, vol. 10, pp. 807–821.
Lim, T.K., Modified stems, roots, bulbs, in Edible Medicinal and Non-Medicinal Plants, The Netherlands: Springer, 2015, pp. 124–203.
Lin, A.I., Zhao-Hu, L.I., Jian-Min, L.I., Xiao-Li, T., Bao-Min, W., Zhi-Xi, Z., and Liu-Sheng, D., Physiological effects of coronatine on seed germination of upland and lowland rice, Acta Agric., 2008, vol. 22, pp. 443–446.
Liu, Y., Zhou, Y., Huang, G., Zhu, N., Li, Z., Zhang, M., and Duan, L., Coronatine inhibits mesocotyl elongation by promoting ethylene production in etiolated maize seedlings, Plant Growth Regul., 2020, vol. 90, pp. 51–61.
Marrelli, M., Amodeo, V., Statti, G., and Conforti, F., Biological properties and bioactive components of Allium cepa L.: focus on potential benefits in the treatment of obesity and related comorbidities, Molecules, 2019, vol. 24, pp. 119–136.
Matysik, J., Alia-Bhalu, B., and Mohanty, P., Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants, Curr. Sci., 2002, vol. 82, pp. 525–532.
Melotto, M., Underwood, W., and He, S.Y., Role of stomata in plant innate immunity and foliar bacterial disease, Annu. Rev. Phytopathol., 2008, vol. 46, pp. 101–122.
Munns, R. and Tester, M., Mechanisms of salinity tolerance, Annu. Rev. Plant Biol., 2008, vol. 59, pp. 651–681.
Norppa, H. and Falck, G.C., What do human micronuclei contain?, Mutagenesis., 2003, vol. 18, pp. 221–233.
Odjegba, V.J. and Adeniran, R.A., Bentazone herbicide induces genotoxic effect and physiological disorders in non-targeted Allium cepa L., Indian J. Plant Physiol., 2015, vol. 20, pp. 375–379.
Peruzzi, L., Carta A., and Altinordu, F., Chromosome diversity and evolution in Allium (Allioideae, Amaryllidaceae), Plant Biosyst., 2017, vol. 151, pp. 212–220.
Saunders, W.S., Shuster, M., Huang, A., Gharaibeh, B., Enyenihi, A.H., Petersen, I., and Gollin, S.M., Chromosomal instability and cytoskeletal defects in oral cancer cells, Proc. Natl. Acad. Sci. U. S. A., 2000, vol. 97, pp. 303–308.
Sharma, P.C. and Gupta, P.K., Karyotypes in some pulse crops, Nucleus, 1982, vol. 25, pp. 181–185.
Soares, A.M.S., Souza, T.F., Jacinto, T., and Machado, O.L.T., Effect of methyl jasmonate on antioxidative enzyme activities and on the contents of ROS and H2O2 in Ricinus communis leaves, Braz. J. Plant Physiol., 2010, vol. 22, pp. 151–158.
Srivastava, A.K. and Singh, D., Assessment of malathion toxicity on cytophysiological activity, DNA damage and antioxidant enzymes in root of Allium cepa model, Sci. Rep., 2020, vol. 10, pp. 1–10.
Schuler, G., Mithofer, A., Baldwin, I.T., Berger, S., Ebel, J., Santos, J.G., Herrmann, G., Holscher, D., Kramell, R., Kutchan, T.M., Maucher, H., Schneider, B., Stenzel, I., Wasternack, C., and Boland, W., Coronalon: a powerful tool in plant stress physiology, FEBS Lett., 2004, vol. 563, pp. 17–22.
Tedesco, S.B. and Laughinghouse, I.V.H.D., Boindicator of genotixicity. The Allium cepa test, J. Environ. Contam., 2012, pp. 138–156.
Thakur, A., Sharma, V., and Thakur, A., Phytotoxin: a mini review, J. Pharmacogn. Phytochem., 2018, vol. 7, pp. 2705–2708.
Turkoglu, S., Genotoxicity of five food preservatives tested on root tips of Allium cepa L., Mutat. Res., 2007, vol. 626, pp. 4–14.
Unyayar, S., Celik, A., Cekic, F.O., and Gozel, A., Cadmium-induced genotoxicity, cytotoxicity and lipid peroxidation in Allium sativum and Vicia faba, Mutagenesis, 2006, vol. 21, pp. 77–81.
Uppalapati, S.R., Ayoubi, P., Weng, H., Palmer, D.A., Mitchell, R.E., Jones, W., and Bender, C.L., The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato, Plant J., 2005, vol. 42, pp. 201–217.
Uppalapati, S.R., Yasuhiro, I., Tamding, W., Ewa, U.W., Takako, I., Kirankumar, S.M., and Bender, C.L., Pathogenicity of Pseudomonas syringae pv. tomato on tomato seedlings: phenotypic and gene expression analyses of the virulence function of coronatine, Mol. Plant-Microbe Interact., 2008, vol. 21, pp. 383–395.
Wang, B.Q., Li, Z.H., Eneji, A.E., Tian, X.L., Zhai, Z.X., Li, J.M., and Duan, L.S., Effects of coronatine on growth, gas exchange traits, chlorophyll content, antioxidant enzymes and lipid peroxidation in maize (Zea mays L.) seedlings under simulated drought stress, Plant Prod. Sci., 2008, vol. 11, pp. 282–290.
Wang, X.S. and Han, J.G., Changes in proline content, activity, and active isoforms of antioxidative enzymes in two alfalfa cultivars under salt stress, Agric. Sci. China., 2009, vol. 8, pp. 431–440.
Xie, Z.X., Duan, L.S., Li, Z.H., Wang, X.D., and Liu, X.J., Dose-dependent effects of coronatine on cotton seedling growth under salt stress, J. Plant Growth Regul., 2015, vol. 34, pp. 651–664.
Xu, J., Zhou, Y., Xu, Z., Chen, Z., and Duan, L., Combining physiological and metabolomic analysis to unravel the regulations of coronatine alleviating water stress in tobacco (Nicotiana tabacum L.), Biomolecules, 2020, vol. 10, pp. 99–114.
Yalcın, E., Macar, O., Kalefetoglu Macar, T., Cavusoglu, D., and Cavusoglu, K., Multi-protective role of Echinacea purpurea L. water extract in Allium cepa L. against mercury(II) chloride, ESPR, 2021, vol. 28, no. 44, pp. 62868–62876.
Yarsan, E., Lipid peroxidation event and its applications for prevention, Van Vet. J., 2014, vol. 9, pp. 89–95.
Zhou, Y.L., Irene, V.V., Robert, C.S., Corne, M.J.P., and Saskia, C.M.V.W., Atmospheric CO2 alters resistance of Arabidopsis to Pseudomonas syringae by affecting abscisic acid accumulation and stomatal responsiveness to coronatine, Front. Plant Sci., 2017, vol. 8, pp. 700–713.
Zou, J., Yue, J., Jiang, W., and Liu, D., Effects of cadmium stress on root tip cells and some physiological indexes in Allium cepa var. agrogarum L., Acta Biol. Cracov. Ser. Bot., 2012, vol. 54, pp. 129–141.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work. All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
The authors confirm that the manuscript has been read and approved by all authors. The authors declare that this manuscript has not been published and not under consideration for publication elsewhere. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Çavuşoğlu, D., Çavuşoğlu, K. Determination of Dose-Dependent Toxic Effect of Coronatine, a Bacterial Phytotoxin, with the Help of Physiological, Cytogenetic, Biochemical, and Anatomical Parameters Using the Allium cepa Test Model. Biol Bull Russ Acad Sci 50, 1081–1092 (2023). https://doi.org/10.1134/S1062359023602124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359023602124