Abstract
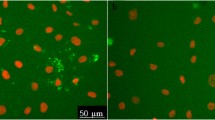
The aim of our research was to study the influence of hydrogen peroxide on the exocytosis of von Willebrand factor (vWF) in human umbilical vein endothelial cells (HUVEC). We have found that H2O2 at a non-toxic concentration (100 μM) increases the amount of vWF secreted by HUVEC by 43 ± 14% over control (p < 0.03) and elevates total exposition of vWF on cell surface by 89 ± 5% (p < 0.01). Analysis of immunofluorescent images of HUVEC with CellProfiler program revealed that the average number of antigen positive structures on the single cell surface increases from 11.4 ± 0.16 in control up to 17.5 ± 0.21 after incubation with H2O2 (p < 0.01). vWF is exposed on the cell surface as dots with the average sizes around 1–3 μm. H2O2 causes an increase in the number of these dots and the appearence of the strings of vWF which are absent in control HUVEC. It is suggested that H2O2 may serve as a messenger which stimulates vWF exocytosis.
Similar content being viewed by others
References
Bernardo, A., Ball, C., Nolasco, L., Moake, J.F., and Dong, J.F., Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow, Blood, 2004, vol. 104, no. 1, pp. 100–106.
Bray, M.A., Vokes, M.S., and Carpenter, A.E., Using Cell- Profiler for automatic identification and measurement of biological objects in images, Curr. Protoc. Mol. Biol., 2015, vol. 109, pp. 1–18.
Breton-Romero, R. and Lamas, S., Hydrogen peroxide signaling in vascular endothelial cells, Redox Biol., 2014, vol. 2, pp. 529–534.
Chen, L., Xiao, J., Kuroda, J., Ago, T., Sadoshima, J., Cohen, R.A., and Tong, X., Both hydrogen peroxide and transforming growth factor beta 1 contribute to endothelial Nox4 mediated angiogenesis in endothelial Nox4 transgenic mouse lines, Biochim. Biophys. Acta, 2014, vol. 1842, no. 12, pp. 2489–2499.
Evangelista, A.M., Thompson, M.D., Bolotina, V.M., Tong, X., and Cohen, R.A., Nox4- and Nox2-dependent oxidant production is required for VEGF-induced SERCA cysteine-674 S-glutathiolation and endothelial cell migration, Free Radic. Biol. Med., 2012, vol. 53, no. 12, pp. 2327–2334.
Ewenstein, B.M., Warhol, M.J., Handin, R.I., and Pober, J.S., Composition of the von Willebrand factor storage organelle (Weibel-Palade body) isolated from cultured human umbilical vein endothelial cells, J. Cell Biol., 1987, vol. 104, no. 5, pp. 1423–1433.
Goncharov, N.V., Sakharov, I., Danilov, S.M., and Sakandelidze, O.G., Use of collagenase from the hepatopancreas of the Kamchatka crab for isolating and culturing endothelial cells of the large vessels in man, Biull. Eksp. Biol. Med., 1987, vol. 104, no. 9, pp. 376–378.
Hughes, S.F., Cotter, M.J., Evans, S.A., Jones, K.P., and Adams, R.A., Role of leucocytes in damage to the vascular endothelium during ischaemia-reperfusion injury, Br. J. Biomed. Sci., 2006, vol. 63, no. 4, pp. 166–170.
Jaffe, E.A., Nachman, R.L., Becker, C.G., and Minick, C.R., Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria, J. Clin. Invest., 1973, vol. 52, no. 11, pp. 2745–2756.
Jones, D.P., Radical-free biology of oxidative stress, Am. J. Physiol. Cell. Physiol., 2008, vol. 295, no. 4, pp. C849–C868.
Kanaji, S., Fahs, S.A., Shi, Q., Haberichter, S.L., and Montgomery, R.R., Contribution of platelet vs. endothelial vwf to platelet adhesion and hemostasis, J. Thromb. Haemost., 2012, vol. 10, no. 8, pp. 1646–1652.
Kudryavtsev, I.V., Garnyuk, V.V., Nadeev, A.D., and Goncharov, N.V., Hydrogen peroxide modulates expression of surface antigens by human umbilical vein endothelial cells in vitro, Biochemistry (Moscow) Supp. S. A: Membr. Cell Biol., 2014, vol. 8, no. 1, pp. 907–912.
Lowenstein, C.J., Morrell, C.N., and Yamakuchi, M., Regulation of Weibel–Palade body exocytosis, Trends Cardiovasc. Med., 2005, vol. 15, no. 8, pp. 302–308.
Maciag, T., Cerundolo, J., Ilsley, S., Kelley, P.R., and Forand, R., An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization, Proc. Natl. Acad. Sci. U. S. A., 1979, vol. 76, no. 11, pp. 5674–5678.
Matsushita, K., Morrell, C.N., Mason, R.J., Yamakuchi, M., Khanday, F.A., Irani, K., and Lowenstein, C.J., Hydrogen peroxide regulation of endothelial exocytosis by inhibition of N-ethylmaleimide sensitive factor, J. Cell Biol., 2005, vol. 170, no. 1, pp. 73–79.
Moake, J.L., Thrombotic microangiopathies, N. Engl. J. Med., 2002, vol. 347, no. 8, pp. 589–600.
Mourik, M.J., Valentijn, J.A., Voorberg, J., Koster, A.J., Valentijn, K.M., and Eikenboom, J., Von Willebrand factor remodeling during exocytosis from vascular endothelial cells, J. Thromb. Haemost., 2013, vol. 11, no. 11, pp. 2009–2019.
Nadeev, A.D., Kudryavtsev, I.V., Serebriakova, M.K., Avdonin, P.V., Zinchenko, V.P., and Goncharov, N.V., Dual proapoptotic and pronecrotic effect of hydrogen peroxide on human umbilical vein endothelial cells, Tsitologiia, 2015, vol. 57, no. 12, pp. 909–916.
Panieri, E. and Santoro, M.M., ROS signaling and redox biology in endothelial cells, Cell Mol. Life Sci., 2015, vol. 72, no. 17, pp. 3281–3303.
Piovella, F., Nalli, G., Malamani, G.D., Majolino, I., Frassoni, F., Sitar, G.M., Ruggeri, A., Dell’Orbo, C., and Ascari, E., The ultrastructural localization of factor VIIIantigen in human platelets, megakaryocytes and endothelial cells utilizing a ferritin-labelled antibody, Br. J. Haematol., 1978, vol. 39, no. 2, pp. 209–213.
Ray, R., Murdoch, C.E., Wang, M., Santos, C.X., Zhang, M., Alom-Ruiz, S., Anilkumar, N., Ouattara, A., Cave, A.C., Walker, S.J., Grieve, D.J., Charles, R.L., Eaton, P., Brewer, A.C., and Shah, A.M., Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo, Arterioscler. Thromb. Vasc. Biol., 2011, vol. 31, no. 6, pp. 1368–1376.
Rondaij, M.G., Bierings, R., Kragt, A., van Mourik, J.A., and Voorberg, J., Dynamics and plasticity of Weibel–Palade bodies in endothelial cells, Arterioscler. Thromb. Vasc. Biol., 2006, vol. 26, no. 5, pp. 1002–1007.
Sadler, J.E., Biochemistry and genetics of von Willebrand factor, Ann. Rev. Biochem., 1998, vol. 67, pp. 395–424.
Shappell, S.B., Toman, C., Anderson, D.C., Taylor, A.A., Entman, M.L., and Smith, C.W., Mac-1 (CD11b/CD18) mediates adherence-dependent hydrogen peroxide production by human and canine neutrophils, J. Immunol., 1990, vol. 144, no. 7, pp. 2702–2711.
Takac, I., Schroder, K., and Brandes, R.P., The Nox family of NADPH oxidases: friend or foe of the vascular system?, Curr. Hypertens. Rep., 2012, vol. 14, no. 1, pp. 70–78.
Turner, N., Nolasco, L., and Moake, J., Generation and breakdown of soluble ultralarge von Willebrand factor multimers, Semin. Thromb. Hemost., 2012, vol. 38, no. 1, pp. 38–46.
Valentijn, K.M., van Driel, L.F., Mourik, M.J., Hendriks, G.J., Arends, T.J., Koster, A.J., and Valentijn, J.A., Multigranular exocytosis of Weibel–Palade bodies in vascular endothelial cells, Blood, 2010, vol. 116, no. 10, pp. 1807–1816.
Vischer, U.M., Von willebrand factor, endothelial dysfunction, and cardiovascular disease, J. Thromb. Haemost, 2006, vol. 4, no. 6, pp. 1186–1193.
Vischer, U.M., Jornot, L., Wollheim, C.B., and Theler, J.M., Reactive oxygen intermediates induce regulated secretion of von Willebrand factor from cultured human vascular endothelial cells, Blood, 1995, vol. 85, no. 11, pp. 3164–3172.
Wagner, D.D. and Bonfanti, R., Von Willebrand factor and the endothelium, Mayo Clin. Proc., 1991, vol. 66, no. 6, pp. 621–627.
Wagner, D.D., Olmsted, J.B., and Marder, V.J., Immunolocalization of von Willebrand protein in Weibel–Palade bodies of human endothelial cells, J. Cell Biol., 1982, vol. 95, no. 1, pp. 355–360.
Wang, H., Yang, Z., Jiang, Y., and Hartnett, M.E., Endothelial NADPH oxidase 4 mediates vascular endothelial growth factor receptor 2-induced intravitreal neovascularization in a rat model of retinopathy of prematurity, Mol. Vis., 2014, vol. 20, рр. 231–241.
Weibel, E.R. and Palade, G.E., New cytoplasmic components in arterial endothelia, J. Cell Biol., 1964, vol. 23, pp. 101–112.
Xiong, Y., Huo, Y., Chen, C., Zeng, H., Lu, X., Wei, C., Ruan, C., Zhang, X., Hu, Z., Shibuya, M., and Luo, J., Vascular endothelial growth factor (VEGF) receptor-2 tyrosine 1175 signaling controls VEGF-induced von Willebrand factor release from endothelial cells via phospholipase C-gamma 1- and protein kinase A-dependent pathways, J. Biol. Chem., 2009, vol. 284, no. 35, pp. 23217–23224.
Yang, D., Liu, X., Liu, M., Chi, H., Liu, J., and Han, H., Protective effects of quercetin and taraxasterol against H2O2-induced human umbilical vein endothelial cell injury in vitro, Exp. Ther. Med., 2015, vol. 10, no. 4, pp. 1253–1260.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © P.V. Avdonin, A.A. Tsitrina, G.Y. Mironova, P.P. Avdonin, I.L. Zharkikh, A.D. Nadeev, N.V. Goncharov, 2017, published in Izvestiya Akademii Nauk, Seriya Biologicheskaya, 2017, No. 5, pp. 549–556.
Rights and permissions
About this article
Cite this article
Avdonin, P.V., Tsitrina, A.A., Mironova, G.Y. et al. Hydrogen peroxide stimulates exocytosis of von Willebrand factor in human umbilical vein endothelial cells. Biol Bull Russ Acad Sci 44, 531–537 (2017). https://doi.org/10.1134/S106235901705003X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106235901705003X