Abstract
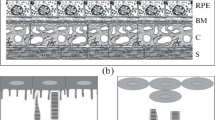
Retinal pigment epithelium (RPE) cells that have the unique ability to reprogram retinal cells in vivo were analyzed in the adult newt. Our own data and that available in the literature on the peculiarities of the biology of these cells (from morphology to molecular profile, which can be associated with the capability of phenotype change) were summarized. It was established that the molecular traits of specialized and poorly differentiated cells are combined in RPE of the adult newt. It was registered that persistent (at a low level) proliferation and rapid change of specific cytoskeleton proteins can contribute to the success of RPE cell reprogramming in the neuronal direction. Each of the considered factors of competence for reprogramming can be found for animal RPE, whose cells are not able in vivo to change the phenotype to a neuronal one; however, their totality (supported by the epigenetic state permissive for conversion) is probably an internal property of only newt RPE.
Similar content being viewed by others
References
Al-Hussaini, Kam, H., Vagler, J.H., et al., Mature retinal pigment epithelium cells are retained in the cell cycle and proliferate in vivo, Mol. Vision, 2008, vol. 14, pp. 1784–1791.
Aulicino, F., Theka, I., Ombrato, L., et al., Temporal perturbation of the Wnt signaling pathway in the control of cell reprogramming is modulated by TGFI, Stem Cell Rep., 2014, vol. 2, pp. 707–720.
Avdonin, P.P., Markitantova, Yu.V., Zinov’eva, R.D., and Mitashov, V.I., Expression of regulatory genes Pax6, Otx2, Six3, and FGF2 during newt retina regeneration, Biol. Bull. (Moscow), 2008, vol. 35, no. 4, pp. 355–362.
Avdonin, P.P., Expression of regulatory genes Fgf2, Pax6, Six3, Otx2, Pitx1, and Pitx2 in epimorphic regeneration of the retina in the newt Pleurodeles waltl, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Moscow: IBR RAN, 2010.
Avdonin, P.P., Grigoryan, E.N., and Markitantova, Yu.V., Transcriptional factor Pitx2: localization during triton retina regeneration, Biol. Bull. (Moscow), 2010, vol. 37, no. 3, pp. 231–236.
Beekman, Ch., Nichane, M., De Clercq, S., et al., Evolutionarily conserved role of nucleostemin: controlling proliferation of stem/progenitor cells during early vertebrate development, Mol. Cell. Biol., 2006, vol. 26, no. 24, pp. 9291–9301.
Bharti, K., Nguyen, M.-T.T., Skuntz, S., et al., The other pigment cell: specification and development of the pigmented epithelium of the vertebrate eye, Pigment Cell Res., 2006, vol. 19, no. 5, pp. 380–394.
Bharti, K., Miller, S.S., and Arnheiter, H., The new paradigm: retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells, Pigment Cell Melanoma Res., 2011, vol. 24, no. 1, pp. 21–34.
Bharti, K., Gasper, M., Ou, J., Brucato, M., et al., A regulatory loop involving PAX6, MITF, and WNT signaling controls retinal pigment epithelium development, PLoS Genet., 2012, vol. 8, no. 7, p. e1002757.
Bonilha, V.L., Retinal pigment epithelium (RPE) cytoskeleton in vivo and in vitro, Exp. Eye Res., 2013. pii: S0014-4835(13)00281-9
Burke, J.M., Epithelial phenotype and the RPE: is the answer blowing in the WNT?, Prog. Ret. Eye Res., 2008, vol. 27, pp. 579–595.
Chiba, C. and Mitashov, V.I., Cellular and molecular events in the adult newt retinal regeneration, in The Strategies for Retinal Tissue Repair and Regeneration in Vertebrates: From Fish to Human, Chiba, C., Ed., Kerala, India: Res. Signpost., 2007, pp. 15–33.
Chiba, C., Hoshino, A., Nakamura, K., et al., Visual cycle protein RPE65 persists in new retinal cells during retinal regeneration of adult newt, J. Comp. Neurol., 2006, vol. 495, no. 4, pp. 391–407.
Crowford, B.J. and Vielkind, U., Location and possible function of fibronectin and laminin in clones of chick retinal pigmented epithelial cells, In vitro 1985, vol. 21, pp. 79–87.
Durairaj, Ch., Chastain, J.E., and Kompella, U.B., Intraocular distribution of melanin in human, monkey, rabbit, minipig and dog eyes, Exp. Eye Res., 2012, vol. 98, no. 1, pp. 23–27.
Eguchi, G., “Transdifferentiation” in pigmented epithelial cells of vertebrate eyes in vitro, in Mech. Cell Change, Ebert, J.D. and Okada, T.S., Eds., New York: J. Wiley and Sons, 1979, pp. 273–291.
Eguchi, G., Lens transdifferentiation in the vertebrate retinal pigmented epithelial cell, Prog. Ret. Res. Pergamon Press Ltd., 1993, vol. 2,ch. 9, pp. 205–230.
Engelhardt, M., Bogdahn, U., and Aigner, L., Adult retinal pigment epithelium cells express neural progenitor properties and the neuronal precursor protein doublecortin, Brain Res., 2005, vol. 1040, pp. 98–111.
Enzmann, V., Howard, R.M., Yamauchi, Y., et al., Enhanced induction of RPE lineage markers in pluripotent neural stem cells engrafted into the adult rat subretinal space, Invest. Ophthalmol. Vis. Sci., 2003, vol. 44, no. 12, pp. 5417–5422.
Fischer, A.J. and Reh, T.A., Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens, Dev. Biol., 2000, vol. 220, pp. 197–210.
Fujimura, N., Taketo, M.M., Mori, M., et al., Spatial and temporal regulation of WNT/beta-catenin signaling is essential for development of the retinal pigment epithelium, Dev. Biol., 2009, vol. 334, pp. 31–45.
Georgiadis, A., Tschernutter, M., Bainbridge, J.W.B., et al., The tight junction associated signaling proteins ZO-1 and ZONAB regulate retinal pigment epithelium homeostasis in mice, PLoS One, 2010, vol. 5, no. 12, p. e15730.
Grigoryan, E.N., Complete retinal detachment causes changes in the expression of cytokeratins in retinal pigment epithelium cells in newts, Izv. Akad. Nauk, Ser. Biol., 1995, no. 4, pp. 412–421.
Grigoryan, E.N., Cytological bases of transdifferentiation in eye tissues of vertebrates, Extended Abstract of Doctoral (Biol.) Dissertation, Moscow: IBR RAN, 1998.
Grigoryan, E., Shared triggering mechanisms of retinal regeneration in lower vertebrates and retinal rescue in higher ones, in Tissue Regeneration—From Basic Biology to Clinical Application, Davies, J., Ed., Rijeka: In Tech. Croatia, 2012, pp. 145–164.
Grigoryan, E.N. and Anton, H.J., The appearance and distribution of the NF-200 neurofilament protein in transdifferentiating cells of the pigment epithelium and in other eye cells during retinal regeneration in newts, Ontogenez, 1993, vol. 24, no. 4, pp. 39–52.
Grigoryan, E.N. and Anton, H.J., An analysis of keratin expression in the cells of the retinal pigment epithelium during transdifferentiation in newts, Ontogenez, 1995, vol. 26, no. 4, pp. 310–323.
Grigoryan, E.N. and Mitashov, V.I., Radioautographic study of proliferation and melanin synthesis in pigment epithelium cells during eye regeneration in newts, Ontogenez, 1979, vol. 10, no. 2, pp. 137–144.
Grigoryan, E.N., Dol’nikova, A.E., and Belkin, V.M., Fibronectin distribution during the transdifferentiation and proliferation of eye cells after retinal detachment and removal of the crystalline lens in newts, Ontogenez, 1990, vol. 21, no. 4, pp. 403–408.
Grigoryan, E.N., Novikova, Yu.P., Kilina, O.V., and Filippov, P.P., A new method of in vitro culturing of retinal pigment epithelium in the posterior eye sector of an adult rat, Klet. Tekhnologii v biologii i meditsine, 2007, no. 4, pp. 207–215.
Grigoryan, E.N., Markitantova, Yu.V., Avdonin, P.P., and Radugina, E.A., Study of regeneration in amphibians in age of molecular-genetic approaches and methods, Russ. J. Genet., 2013, vol. 49, no. 1, pp. 46–62.
Hausman, R.E., Ocular extracellular matrices in development, Prog. Ret. Eye Res., 2007, vol. 26, no. 2, pp. 162–188.
Hiscott, P., Sheridan, C., Magee, R.M., and Grierson, I., Matrix and the retinal pigment epithelium in proliferative retinal disease, Prog. Ret. Eye Res., 1999, vol. 18, no. 2, pp. 167–190.
Ikegami, Y., Mitsuda, S., and Araki, M., Neural cell differentiation from retinal pigment epithelial cells of the newt: an organ culture model for the urodele retinal regeneration, J. Neurobiol., 2002, vol. 50, pp. 209–220.
Keefe, J.R., An analysis of urodelian retinal regeneration, J. Exp. Zool., 1973, vol. 184, pp. 185–257.
Kelaini, S., Cochrane, A., and Margariti, A., Direct repro-gramming of adult cells: avoiding the pluripotent state, Stem Cells Cloning, 2014, vol. 7, pp. 19–29.
Kiskinis, E. and Eggan, K., Progress toward the clinical application of patient-specific pluripotent stem cells, J. Clin. Invest., 2010, vol. 120, no. 1, pp. 51–59.
Korte, G.E., Perlman, J.I., and Pollak, A., Regeneration of mammalian retinal pigment epithelium, Int. Rev. Cytol., 1994, vol. 152, pp. 223–263.
Kuznetsova, A.V., Grigoryan, E.N., and Aleksandrova, M.A., Adult human retinal pigment epithelial cells—a potential source of cells for regeneration retina, Tsitologiia, 2011, vol. 53, no. 6, pp. 505–512.
Von Leithner, P.L., Ciurtin, C., and Jeffery, J., Microscopic mammalian retinal pigment epithelium lesions induce widespread proliferation with differences in magnitude between center and periphery, Mol. Vision, 2010, vol. 16, pp. 570–581.
Luz-Madrigal, A., Grajales-Esquivel, E., McCorkle, A., et al., Reprogramming of the chick retinal pigmented epithelium after retinal injury, BMC Biol., 2014, vol. 12, p. 28.
Ma, H. and Pederson, T., Nucleostemin: a multiplex regulator of cell-cycle progression, Trends Cell Biol., 2008, vol. 18, no. 12, pp. 575–579.
Machalinska, A., Kawa, M.P., Pius-Sadowska, E., et al., Endogenous regeneration of damaged retinal pigment epithelium following low dose sodium iodate administration: an insight into the role of glial cells in retinal repair, Exp. Eye Res., 2013, vol. 112, pp. 68–78.
Maki, N., Takechi, K., Sano, S., et al., Rapid accumulation of nucleostemin in nucleolus during newt regeneration, Dev. Dynam., 2007, vol. 263, pp. 941–950.
Maki, N., Suetsugu-Maki, R., Tarui, H., et al., Expression of stem cell pluripotency factors during regeneration in newts, Dev. Dynam., 2009, vol. 238, no. 6, pp. 1613–1616.
Markitantova, Yu.V., Avdonin, P.P., and Grigoryan, E.N., FGF2 signaling pathway components in tissues of the posterior eye sector in the adult newt Pleurodeles waltl, Biol. Bull. (Moscow), 2014a, vol. 41, no. 4, pp. 297–305.
Markitantova, Yu.V., Avdonin, P.P., and Grigoryan, E.N., Nucleostemin expression during in situ reprogramming of eye pigment epithelium cells during retina regeneration in an adult newt, Cell Tissue Biol., 2014b (in press).
Martinez-Morales, J.R., Dolez, V., Rodrigo, I., et al., Otx2 activates the molecular network underlying retinal pigment epithelium differentiation, J. Biol. Chem., 2003, vol. 278, pp. 21721–21731.
Martinez-Morales, J.R., Del Bene, F., Nica, G., et al., Differentiation of the vertebrate retina is coordinated by an FGF signaling center, Dev. Cell, 2005, vol. 8, no. 4, pp. 565–574.
Milyushina, L.A., Verdiev, B.I., Kuznetsova, A.V., and Aleksandrova, M.A., Expression of pluripotent and retinal markers in pigment retinal epithelium in adult human eye in vitro, Klet. Tekhnol. Biol. Med., 2012, no. 1, pp. 44–50.
Mitashov, V.I., Patterns of changes in mitotic cycles during cell transformation and regeneration in lower vertebrates, Tsitologiia, 1980, vol. 2, pp. 371–380.
Mitashov, V.I., Retinal regeneration in amphibians, Int. J. Dev. Biol., 1997, vol. 41, no. 6, pp. 893–905.
Mitashov, V.I., Expression of regulatory and tissue-specific genes controlling regenerative potencies of eye tissues in vertebrates, Russ. J. Dev. Biol., 2007, vol. 38, no. 4, pp. 198–205.
Mitashov, V.I., Arsanto, J.P., Markitantova, Y.V., and Thouveny, Y., Remodeling processes during neural retinal regeneration in adult urodeles: an immunohistochemical survey, Int. J. Dev. Biol., 1995, vol. 39, no. 6, pp. 993–1003.
Mizuno, A., Yasumuro, H., Yoshikawa, T., et al., MEK-ERK signaling in adult newt retinal pigment epithelium cells is strengthened immediately after surgical induction of retinal regeneration, Neurosci. Lett., 2012, vol. 523, no. 1, pp. 39–44.
Mohan, P.S. and Spiro, R.G., Macromolecular organization of basement membranes, J. Biol. Chem., 1986, vol. 261, pp. 4328–4336.
Moiseyev, G., Chen, Y., Takahashi, Y., et al., PE65 is the isomerohydrolase in the retinoid visual cycle, Proc. Natl. Acad. Sci. U.S.A., 2005, vol. 102, no. 35, pp. 12413–12418.
Muller, F., Rohrer, H., and Vogel-Hopker, A., Bone morphogenetic proteins specify the retinal pigment epithelium in the chick embryo, Development, 2007, vol. 134, pp. 3483–3493.
Nishihara, D., Yajima, I., Tabata, H., et al., Otx2 is involved in the regional specification of the developing retinal pigment epithelium by preventing the expression of Sox2 and Fgf8, factors that induce neural retina differentiation, PLoS One, 2012, vol. 7, no. 11, p. e48879.
Novikova, Yu.P., Poplinskaya, V.A., Aleinikova, K.S., and Grigoryan, E.N., A study of the localization and accumulation of S-phase cells in the retina of newt Pleurodeles waltl after experimental pigment epithelial detachment, Russ. J. Dev. Biol., 2008, vol. 39, no. 2, pp. 116–121.
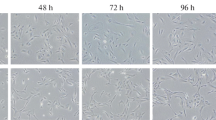
Novikova, Yu.P., Aleinikova, K.S., Krasnov, M.S., et al., The retinal pigment epithelial cells of the adult newt and rat under conditions of in vitro organotypic culture, Biol. Bull. (Moscow), 2010, vol. 37, no. 3, pp. 221–230.
Novikova, Yu.P., Identification and activation in vitro of hidden regenerative potentials of the retina of vertebrate eye, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Moscow: IBR RAN, 2010.
Okada, T.S., Transdifferentiation: Flexibility in Cell Differentiation, Oxford: Clarendon Press, 1991.
Ortiz, J.R., Vigny, M., Courtois, Y., and Jeanny, J.-C., Immunocytochemical study of extracellular matrix components during lens and neural retina regeneration in the adult newt, Exp. Eye Res., 1992, vol. 54, no. 6, pp. 861–870.
Park, C.M., Basic fibroblast growth factor induces retinal regeneration in vivo, Dev. Biol., 1989, vol. 134, pp. 201–205.
Phillips, M.J., Wallace, K.A., Dickerson, S.J., et al., Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses, Invest. Ophthalmol. Vis. Sci., 2012, vol. 53, no. 4, pp. 2007–2019.
Rattner, A., Toulabi, L., Williams, J., et al., The genomic response of the retinal pigment epithelium to light damage and retinal detachment, J. Neurosci., 2008, vol. 28, no. 39, pp. 9880–9889.
Reh, T.A., Nagy, T., and Gretton, H., Retinal pigmented epithelial cells induced to transdifferentiate to neurons by laminin, Nature, 1987, vol. 330, no. 6143, pp. 68–71.
Sakami, S., Hisatomi, O., Sakakibara, S., et al., Downregulation of Otx2 in the dedifferentiated RPE cells of regenerating newt retina, Brain Res. Dev. Brain Res., 2005, vol. 155, no. 1, pp. 49–59.
Sakami, S., Etter, P., and Reh, T.A., Activin signaling limits the competence for retinal regeneration from the pigmented epithelium, Mech. Dev., 2008, vol. 125, pp. 106–116.
Salero, E., Blenkinsop, T.A., Corneo, B., et al., Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives, Cell Stem Cell, 2012, vol. 10, p. 88–95.
Schmidt, S.Y. and Peisch, R.D., Melanin concentration in normal human retinal pigment epithelium. Regional variation and age-related reduction, Invest. Ophthalmol. Vis. Sci., 1986, vol. 27, pp. 1063–1067.
Schraermeyer, U. and Heimann, K., Current understanding of the role of retinal pigment epithelium and its pigmentation, Pigment Cell Res., 1999, vol. 12, pp. 219–236.
Spence, J.R., Madhavan, M., Aycinena, K., and Del RioTsonis, K., Retina regeneration in the chick embryo is not induced by spontaneous Mitf downregulation but requires FGF/FGFR/MEK/Erk dependent upregulation of Pax6, Mol. Vis., 2007, vol. 13, pp. 57–65.
Stone, L.S., The role of retinal pigment cells in regenerating neural retinae of adult salamander eyes, J. Exp. Zool., 1950, vol. 113, pp. 9–31.
Stroeva, O.G. and Mitashov, V.I., Retinal pigment epithelium: proliferation and differentiation during development and regeneration, Int. Rev. Cytol., 1983, vol. 83, pp. 221–293.
Susaki, K. and Chiba, Ch., MEK mediates in vitro neural transdifferentiation of the adult newt retinal pigment epithelium cells: is FGF2 an induction factor?, Pigment Cell Res., 2007, vol. 20, pp. 364–379.
Svendsen, C.N., Back to the future: how human induced pluripotent stem cells will transform regenerative medicine, Hum. Mol. Genet., 2013, vol. 22, no. R1, pp. R32–R38.
Tsai, R.Y. and McKay, R.D., A multistep, GTP-driven mechanism controlling the dynamic cycling of nucleostemin, J. Cell Biol., 2005, vol. 168, no. 2, pp. 179–184.
Tsai, R.Y.L. and Meng, L., Nucleostemin: a latecomer with new tricks, Int. J. Biochem. Cell Biol., 2009, vol. 41, no. 11, pp. 2122–2124.
Turksen, K., Aubin, J.E., Sodek, J., and Kalnins, V.I., Changes in the distribution of laminin, fibronectin, type IV collagen and heparan sulfate proteoglycan during colony formation by chick retinal pigment epithelial cells in vitro, Collagen Related Res., 1984, vol. 4, no. 6, pp. 413–426.
Vigneault, F., Zaniolo, K., Gaudreault, M., et al., Control of integrin genes expression in the eye, Prog. Ret. Eye Res., 2007, vol. 26, no. 2, pp. 99–161.
Westenskow, P., Piccolo, S., and Fuhrmann, S., Beta-catenin controls differentiation of the retinal pigment epithelium in the mouse optic cup by regulating Mitf and Otx2 expression, Development, 2009, vol. 136, pp. 2505–2510.
Yoshikawa, T., Mizuno, A., Yasumuro, H., et al., MEKERK and heparin-susceptible signaling pathways are involved in cell-cycle entry of the wound edge retinal pigment epithelium cells in the adult newt, Pigment Cell Melanoma Res., 2012, vol. 25, pp. 66–82.
Zhang, X.M. and Yang, X.J., Temporal and spatial effects of sonic hedgehog signaling in chick eye morphogenesis, Dev. Biol., 2001, vol. 233, pp. 271–290.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.N. Grigoryan, 2015, published in Izvestiya Akademii Nauk, Seriya Biologicheskaya, 2015, No. 1, pp. 5–16.
Rights and permissions
About this article
Cite this article
Grigoryan, E.N. Competence factors of retinal pigment epithelium cells for reprogramming in the neuronal direction during retinal regeneration in newts. Biol Bull Russ Acad Sci 42, 1–11 (2015). https://doi.org/10.1134/S1062359015010045
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359015010045