Abstract
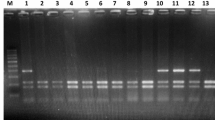
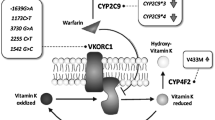
The allele and genotype frequency distribution at polymorphic loci rs1799853 (430C>T) and rs1057910 (A1075C) of the CYP2C9 gene, rs2108622 (1347C>T) of the CYP4F2 gene, and rs9923231 (1639G>A) of the VKORC1 gene in the Buryat population was examined. The study involved 197 volunteers living in the Republic of Buryatia: 124 woman and 73 men, average age of 57.51 ± 10.88 years. Genetic typing of DNA samples was performed by polymerase chain reaction (RT-PCR). The frequency of the minor alleles *2 and *3 of the CYP2C9 gene was 2.03 and 3.05%, respectively. The frequency of the allele T of the CYP4F2 gene was 31.22%; the frequency of the allele A of the VKORC1 gene was 85.28%. The obtained results could be used in the prognosis of pharmacotherapy of warfarin in the population Buryat population.

Similar content being viewed by others
REFERENCES
Goncharova, I.A., Babushkina, N.P., Minaycheva, L.I., et al., Prevalence of alleles of polymorphic variants Leu33Pro and Leu66Arg gene ITGB3 among inhabitants of Siberia Russ. J. Genet., 2013, vol. 49, no. 8, pp. 877—880. https://doi.org/10.1134/S1022795413070053
Kolesnikova, L.I., Bairova, T.A., Pervushina, O.A., et al., Association of (192) Q>R polymorphism of the paraoxonase gene with a lipid profile and components of lipid peroxidation and antioxidant protection in populations of Russians and Buryats from Eastern Siberia, Russ. J. Genet., 2015, vol. 51, no. 2, pp. 193—197. https://doi.org/10.1134/S102279541502009X
Alomar, M.J., Factors affecting the development of adverse drug reactions, Saudi Pharm. J., 2014, vol. 22, no. 2, pp. 83—94. https://doi.org/10.1016/j.jsps.2013.02.003
Mustafina, O.E., Tuktarova, I.A., Karimov, D.D., et al., CYP2D6, CYP3A5, and CYP3A4 gene polymorphisms in Russian, Tatar, and Bashkir populations. Russ. J. Genet., 2015, vol. 51, no. 1, pp. 98—107. https://doi.org/10.1134/S1022795415010081
https://www.fda.gov/drugs/science-and-research-drugs/ table-pharmacogenomic-biomarkers-drug-labeling.
Freedman, M.D., Oral anticoagulants: pharmacodynamics, clinical indications and adverse effects, J. Clin. Pharmacol., 1992, vol. 32, no. 3, pp. 196—209. https://doi.org/10.1002/j.1552-4604.1992.tb03827.x
Altshuler, D. and Donnelly, P., International HapMap Consortium: a haplotype map of the human genome, Nature, 2005, vol. 437, no. 7063, pp. 1299—1320. https://doi.org/10.1038/nature04226
Sawyer, S.L., Mukherjee, N., Pakstis, A.J., et al., Linkage disequilibrium patterns vary substantially among populations, Eur. J. Hum. Genet., 2005, vol. 13, no. 5, pp. 677—686. https://doi.org/10.1038/sj.ejhg.5201368
Makeeva, O., Stepanov, V., Puzyrev, V., et al., Global pharmacogenetics: genetic substructure of Eurasian populations and its effect on variants of drug-metabolizing enzymes, Pharmacogenomics, 2008, vol. 9, no. 7, pp. 847—868. https://doi.org/10.2217/14622416.9.7.847
Sangadeeva, D.Ts., Daindorov, D.S., Pushkarev, B.S., et al., Frequency of VKORC1 genetic polymorphism among Russians and Buryats in the Trans-Baikal Territory, Med. Zavtrashnego Dnya, 2017, pp. 299—300.
Sanderson, S., Emery, J., and Higgins, J., CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuG Enet™ systematic review and meta-analysis, Genet. Med., 2005, vol. 7, no. 2, pp. 97—104.
NIH, Genome Reference Consortium, The International Genome Sample Resource. http://www.1000genomes.org/.
Averin, A.N., Native minorities: development dynamics, Vestn. Beloruss. Gos. Univ., 2015, no. 14, pp. 70—75.
Vasilyev, F.F., Danilova, D.A., Kaimonov, V.S., et al., Frequency distribution of polymorphisms of CYP2C19, CYP2C9, VKORC1 and SLCO1B1 genes in the Yakut population, Res. Pharm. Sci., 2016, vol. 11, no. 3, pp. 259—264.
Korytina, G., Kochetova, O., Akhmadishina, L., et al., Polymorphism of cytochrome P450 genes in three ethnic groups from Russia, Balkan. Med. J., 2012, vol. 29, pp. 252—260. https://doi.org/10.1089/gtmb.2017.0036
Korchagina, R.P., Osipova, L.P., Vavilova, N.A., et al., Genetic polymorphism of drug-metyabolising cytochrome P450 2C9 in native minorities of Northern Siberia, Sib. Nauchn. Med. Zh., 2011, vol. 31, no. 6, pp. 39—46.
Lavrinov, P.A., Belova, N.I., and Vorob’eva, N.A., VKORC1-1639 G/A and 1173 C/T gene polymorphisms in the indigenous population of the Nenets Autonomous Okrug, Uch. Zap. S.-Peterb. Gos. Med. Univ. im. I. P. Pavlova, 2014, vol. 21, no. 2, pp. 33—36.
Shuev, G.N., Sychev, D.A., Suleimanov, S.Sh., et al., Comparison of the polymorphism frequency of CYP2C9, CYP2C19, CYP2D6, ABCB1, SLCO1B1 genes in ethnic groups of Nanai and Russians, Farmakogenet. Farmakogenomika, 2016, no. 2, pp. 12—18.
Bairova, T.A., Novikova, E.A., Belyalov, F.I., et al., Prevalence of polymorphisms of genes of cytochrome P450—warfarin metabolizers—in Eastern Siberia, Acta Biomed. Sci., 2018, vol. 3, no. 5, pp. 39—48. https://doi.org/10.29413/ABS.2018-3.5.6
Sychev, D.A., Rozhkov, A.V., Ananichuk, A.V., and Kazakov, R.E., Evaluation of genotype-guided acenocoumarol dosing algorithms in Russian patients, Drug Metab. Pers. Ther., 2017, vol. 32, no. 2, pp. 109—114. https://doi.org/10.1515/dmpt-2016-0043
Gaikovitch, E.A., Cascorbi, I., Mrozikiewicz, P.M., et al., Polymorphisms of drug-metabolizing enzymes CYP2C9, CYP2C19, CYP2D6, CYP1A1, NAT2 and of P-glycoprotein in a Russian population, Eur. J. Clin. Pharmacol., 2003, vol. 59, no. 4, pp. 303—312. https://doi.org/10.1007/s00228-003-0606-2
Baturin, V.A., Tsarukyan, A.A., and Kolodiichuk, E.V., Study of CYP2C9 gene polymorphism in ethnic groups of the Stavropol krai population, Med. Vestn. Sev. Kavkaza, 2014, vol. 9, no. 1, pp. 45—48.
Dai, D.P., Xu, R.A., Hu, L.M., et al., CYP2C9 polymorphism analysis in Han Chinese populations: building the largest allele frequency database, Pharmacogenomics J., 2014, vol. 14, no. 1, pp. 85—92. https://doi.org/10.1038/tpj.2013.2
Li, S., Zou, Y., Wang, X., et al., Warfarin dosage response related pharmacogenetics in Chinese population, PLoS One, 2015, vol. 10, no. 1. e0116463. https://doi.org/10.1371/journal.pone.0116463
Cen, H.J., Zeng, W.T., Leng, X.Y., et al., CYP4F2 rs2108622: a minor significant genetic factor of warfarin dose in Han Chinese patients with mechanical heart valve replacement, Brit. J. Clin. Pharmacol., 2010, vol. 70, no. 2, pp. 234—240. https://doi.org/10.1111/j.1365-2125.2010.03698.x
Yoshizawa, M., Hayashi, H., Tashiro, Y., et al., Effect of VKORC1–1639 G>A polymorphism, body weight, age, and serum albumin alterations on warfarin response in Japanese patients, Thromb. Res., 2009, vol. 124, no. 2, pp. 161—166. https://doi.org/10.1016/j.thromres.2008.11.011
Sasano, M., Ohno, M., Fukuda, Y., et al., Verification of pharmacogenomics-based algorithms to predict warfarin maintenance dose using registered data of Japanese patients, Eur. J. Clin. Pharmacol., 2019, vol. 75, no. 7, pp. 901—911. https://doi.org/10.1007/s00228-019-02656-7
Yoon, Y.R., Shon, J.H., Kim, M.K., et al., Frequency of cytochrome P450 2C9 mutant alleles in a Korean population, Brit. J. Clin. Pharmacol., 2001, vol. 51, no. 3, pp. 277—280. https://doi.org/10.1046/j.1365-2125.2001.00340.x
Kim, K.A., Song, W.G., Lee, H.M., et al., Multiplex pyrosequencing method to determine CYP2C9* 3, VKORC1* 2, and CYP4F2* 3 polymorphisms simultaneously: its application to a Korean population and comparisons with other ethnic groups, Mol. Biol. Rep., 2014, vol. 41, no. 11, pp. 7305—7312. https://doi.org/10.1007/s11033-014-3617-4
Wen, M.S., Lee, M., Chen, J.J., et al., Prospective study of warfarin dosage requirements based on CYP2C9 and VKORC1 genotypes, Clin. Pharmacol. Ther., 2008, vol. 84, no. 1, pp. 83—89. https://doi.org/10.1038/sj.clpt.6100453
Lee, M.M., Chen, C.H., Chou, C.H., et al., Genetic determinants of warfarin dosing in the Han-Chinese population, Pharmacogenomics, 2009, vol. 10, no. 12, pp. 1905—1913. https://doi.org/10.2217/pgs.09.106
Wattanachai, N., Kaewmoongkun, S., Pussadhamma, B., et al., The impact of non-genetic and genetic factors on a stable warfarin dose in Thai patients, Eur. J. Clin. Pharmacol., 2017, vol. 73, no. 8, pp. 973—980. https://doi.org/10.1007/s00228-017-2265-8
Gan, G.G., Phipps, M.E., Lee, M.M., et al., Contribution of VKORC1 and CYP2C9 polymorphisms in the interethnic variability of warfarin dose in Malaysian populations, Ann. Hematol., 2011, vol. 90, no. 6, pp. 635—641. https://doi.org/10.1007/s00277-010-1119-6
Singh, O., Sandanaraj, E., Subramanian, K., et al., Influence of CYP4F2 rs2108622 (V433M) on warfarin dose requirement in Asian patients, Drug Metab. Pharmacokinet., 2011, vol. 26, no. 2, pp. 130—136. https://doi.org/10.2133/dmpk.DMPK-10-RG-080
Rusdiana, T., Araki, T., Nakamura, T., et al., Responsiveness to low-dose warfarin associated with genetic variants of VKORC1, CYP2C9, CYP2C19, and CYP4F2 in an Indonesian population, Eur. J. Clin. Pharmacol., 2013, vol. 69, no. 3, pp. 395—405. https://doi.org/10.1007/s00228-012-1356-9
Suriapranata, I.M., Tjong, W.Y., Wang, T., et al., Genetic factors associated with patient-specific warfarin dose in ethnic Indonesians, BMC Med. Genet., 2011, vol. 12, no. 1, pp. 80—88. https://doi.org/10.1186/1471-2350-12-80
Kumar, D.K., Shewade, D.G., Loriot, M.A., et al., Effect of CYP2C9, VKORC1, CYP4F2 and GGCX genetic variants on warfarin maintenance dose and explicating a new pharmacogenetic algorithm in South Indian population, Eur. J. Clin. Pharmacol., 2014, vol. 70, no. 1, pp. 47—56. https://doi.org/10.1007/s00228-013-1581-x
Kumar, D.K., Shewade, D.G., Manjunath, S., et al., Inter and intra ethnic variation of vitamin K epoxide reductase complex and cytochrome P450 4F2 genetic polymorphisms and their prevalence in South Indian population, Indian J. Hum. Genet., 2013, vol. 19, no. 3, pp. 301—310. https://doi.org/10.4103/0971-6866.120817
Namazi, S., Azarpira, N., Hendijani, F., et al., The impact of genetic polymorphisms and patient characteristics on warfarin dose requirements: a cross-sectional study in Iran, Clin. Ther., 2010, vol. 32, no. 6, pp. 1050—1060. https://doi.org/10.1016/j.clinthera.2010.06.010
Khosropanah, S., Faraji, S.N., Habibi, H., et al., Correlation between rs2108622 locus of CYP4F2 gene single nucleotide polymorphism and warfarin dosage in Iranian cardiovascular patients, Iran. J. Pharm. Res., 2017, vol. 16, no. 3, pp. 1238—1246.
Kocael, A., Eronat, A.P., Tüzüner, M.B., et al., Interpretation of the effect of CYP2C9, VKORC1 and CYP4F2 variants on warfarin dosing adjustment in Turkey, Mol. Biol. Rep., 2019, vol. 46, no. 2, pp. 1825—1833. https://doi.org/10.1007/s11033-019-04634-9
Spitsyna, N.Kh., Bychkovskaya, L.S., Makarov, S.V., et al., Genetic variation amongst Selkups of the North-Western Siberia, Vestn. Mosk. Gos. Univ., Ser. XXIII. Antropol., 2013, vol. 23, no. 3, pp. 120—126.
Johnson, J.A., Caudle, K.E., Gong, L., et al., Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for pharmacogenetics-guided warfarin dosing: 2017 update, Clin. Pharmacol. Ther., 2017, vol. 102, no. 3, pp. 397—404. https://doi.org/10.1002/cpt.668
Alvarellos, M.L., Sangkuhl, K., Daneshjou, R., et al., PharmGKB summary: very important pharmacogene information for CYP4F2, Pharmacogenet. Genomics, 2015, vol. 25, no. 1, pp. 41—47. https://doi.org/10.1097/FPC.0000000000000100
Caldwell, M.D., Awad, T., Johnson, J.A., et al., CYP4F2 genetic variant alters required warfarin dose, Blood, 2008, vol. 111, no. 8, pp. 4106—4112. https://doi.org/10.1182/blood-2007-11-122010
D’Andrea, G., D’Ambrosio, R.L., Perna, P., et al., A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin, Blood, 2005, vol. 105, no. 2, pp. 645—649. https://doi.org/10.1182/blood-2004-06-2111
Rieder, M.J., Reiner, A.P., Gage, B.F., et al., Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose, N. Engl. J. Med., 2005, vol. 352, no. 22, pp. 2285—2293. https://doi.org/10.1056/NEJMoa044503
Yuan, H.Y., Chen, J.J., Lee, M.M., et al., A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity, Hum. Mol. Genet., 2005, vol. 14, no. 13, pp. 1745—1751. https://doi.org/10.1093/hmg/ddi180
Gage, B.F., Eby, C., Johnson, J.A., et al., Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin, Clin. Pharmacol. Ther., 2008, vol. 84, no. 3, pp. 326—331. https://doi.org/10.1038/clpt.2008.10
Sipeky, C., Csongei, V., Jaromi, L., et al., Vitamin K epoxide reductase complex 1 (VKORC1) haplotypes in healthy Hungarian and Roma population samples, Pharmacogenomics, 2009, vol. 10, no. 6, pp. 1025—1032. https://doi.org/10.2217/pgs.09.46
Sipeky, C., Lakner, L., Szabo, M., et al., Interethnic differences of CYP2C9 alleles in healthy Hungarian and Roma population samples: relationship to worldwide allelic frequencies, Blood Cells, Mol. Dis., 2009, vol. 43, no. 3, pp. 239—242. https://doi.org/10.1016/j.bcmd.2009.05.005
Sipeky, C., Weber, A., Melegh, B.I., et al., Interethnic variability of CYP4F2 (V433M) in admixed population of Roma and Hungarians, Environ. Toxicol. Pharmacol., 2015, vol. 40, no. 1, pp. 280—283. https://doi.org/10.1016/j.etap.2015.05.008
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement of compliance with standards of research involving humans as subjects. All procedures performed in a study involving people comply with the ethical standards of the institutional and/or national committee for research ethics and the 1964 Helsinki Declaration and its subsequent changes or comparable ethical standards. Informed voluntary consent was obtained from each of the participants.
Rights and permissions
About this article
Cite this article
Sambyalova, A.Y., Bairova, T.A., Belyaeva, E.V. et al. CYP2C9, CYP4F2, VKORC1 Gene Polymorphism in Buryat Population. Russ J Genet 56, 1496–1503 (2020). https://doi.org/10.1134/S1022795420120121
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795420120121