Abstract
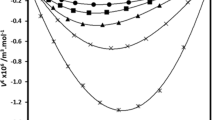
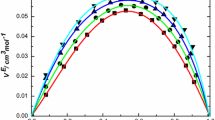
The density (ρ), viscosity (η), and speed of sound (u) were measured for binary mixtures of 3‑methyl-1-butanol(isoamylalcohol) with 1,2-dichloroethane (DCE), 1,1,2-trichloroethane (TCE), and 1,1,2,2-tetrachloroethane (TECE) over the entire range of mole fractions and at temperature 298.15, 303.15, and 308.15 K. From these measurements, the values of excess molar volume (VE), deviation in viscosity (∆η), deviation in isentropic compressibility (∆ks), excess Gibbs free energy of activation of viscous flow (G*E) and Grunberg−Nissan interaction parameter (d ') have been calculated. The excess parameter deviations have been fitted to Redlich−Kister type polynomial equation using multiparametric nonlinear regression analysis to estimate the binary coefficients and standard deviations. The results were discussed in terms of molecular interactions and dipole-dipole interactions. The effects of temperature and chlorine atoms number of investigated chloroethanes were also studied for the binary mixtures.






Similar content being viewed by others
REFERENCES
C. L. Lafuente, B. Giner, A. Villares, I. Gascon, and P. Cea, Int. J. Tthermophys. 25, 1735 (2004).
P. S. Nikam, L. N. Shirsat, and M. Hasan, J. Indian. Chem. Soc. 77, 244 (2000).
E. Perez, M. Cardoso, A. M. Mainar, J. L. Pardo, and J. S. Uricta, J. Chem. Eng. Data 48, 1306 (2003).
R. L. L. Venkatramana, K. Sivakumar, and K. D. Reddy, Fluid Phase Equilib. 367, 7 (2014).
V. Shymala, V. K. Shivakumar, and P. Venkateshwarlu, J. Chem. Thermodyn. 38, 1553 (2006).
T. S. Jyostna and N. Satyanarayana, Ind. J. Chem. Technol. 13, 71 (2006).
M. N. Roy, R. S. Sah, and P. Paradhan, Int. J. Thermophys. 31, 316 (2010).
N. Raghuram, R. Suresh, G. Ramesh, G. Sowjanya, and T. S. Jyostna, J. Therm. Anal. Calorim. 119, 2017 (2015).
K. R. Dayananda, H. Iloukhani, and M. V. P. Rao, Fluid Phase Equilib. 17, 123 (1984).
L. Venkatramana, R. L. Gardas, K. Sivakumar, and K. D. Reddy, Fluid Phase Equilib. 367, 721 (2014).
V. Shymala, K. Shivakumar, and P. Venkateshwarlu, J. Chem. Thermodyn. 38, 1553 (2006).
P. S. Nikam, T. R. Mahale, and M. Hasan, J. Chem. Eng. Data 43, 436 (1998).
K. Sreenivasulu, R. L. Gardas, P. Venkatashwarlu, and K. Sivakumar, J. Chem. Thermodyn. 67, 203 (2006).
B. Satyanarayana, K. B. Ranjith, T. J. Savitha, and N. Satyanarayana, J. Chem. Thermodyn. 39, 16 (2007).
P. S. Nikam, L. N. Shrisat, and M. B. Hasan, J. Chem. Eng. Data 43, 732 (1998).
T. S. Jyostna and N. Satyanarayana, J. Chem. Eng. Data 50, 89 (2005).
L. Grunberg and A. H. Nissan, Mix. Law. Visc. Nature 164, 799 (1949).
P. K. Katti and M. M. Chaudhry, J. Chem. Eng. Data 45, 693 (2000).
M. Alejandra, C. Salvador, C. Alberto, A. Mainar, and P. Miguel, Phys. Chem. Liq. 49, 720 (2011).
O. Redlich and A. T. Kister, Indian Eng. Chem. 40, 345 (1948).
H. Kumar, M. Singla, and A. Khosla, J. Solut. Chem. 42, 428 (2013).
W. L. Wen, L. T. Chang, and I. M. Shiah, J. Chem. Eng. Data 44, 994 (1999).
B. Garcia, S. Aparicio, A. M. Navarro, R. Alcalde, and J. L. Leal, J. Phys. Chem. B 108, 15841 (200).
B. Sathyanarayana, T. S. Jyostna, and N. Sathyanarayana, Indian J. Pure Appl. Phys. 44, 587 (2006).
C. Subhas, R. Bhatia, and P. Gyan, Int. J. Thermophys. 31, 2119 (2101).
R. D. Peralta, R. Infante, G. Cortez, and A. Cisneros, Chem. Eng. Commun. 192, 684 (2005).
N. V. Choudary and P. R. Naidu, Can. J. Chem. 59, 2210 (1981).
M. Chorazewski, J. Chem. Eng. Data 52, 154 (2007).
N. V. Choudary, A. Krishnaiah, and P. R. Naidu, J. Chem. Eng. Data 27, 412 (1982).
N. V. Choudary, J. C. Mouli, and P. R. Naidu, Acoust. Lett. 6, 56 (1982).
S. L. Oswal and I. N. Patel, J. Mol. Liq. 116, 99 (2005).
A. Ali, A. K. Nain, D. Chand, and R. Ahmad, Phys. Chem. Liq. 43, 205 (2005).
G. Larsen, Z. K. Ismal, B. Herreies, and R. D. Perra, J. Phys. Chem. 102, 473 (1919).
H. Iloukhani and B. Samiey, J. Chem. Eng. Data 50, 1911 (2005).
H. Iloukhani, J. B. Parsa, and M. Hatami, Phys. Chem. Liq. 46, 495 (2008).
F. L. B. Raton, Handbook Chem. Phys. 15, 14 (2000).
G. P. Dubey, M. Sharma, and N. Dubey, J. Chem. Thermodyn. 40, 309 (2008).
P. Brocos, A. Pineiro, R. Bravo, and A. Amigo, Phys. Chem. Chem. Phys. 5, 550 (2003).
A. Pineiro, P. Brocos, A. Amigo, M. Pintos, and R. Bravo, Phys. Chem. Liq. 38, 251 (2000).
D. S. Gill and T. S. Cheema, J. Phys. Chem. 134, 205 (1983).
R. J. Fort and W. R. Moore, Trans. Faraday Soc. 62, 1112 (1966).
S. C. Bhatia, R. Bhatia, and G. P. Dubey, J. Mol. Liq. 144, 1639 (2009).
C. Subhash, R. B. Bhatia, and G. P. Dubey, J. Chem. Eng. Data 54, 3303 (2009).
S. C. Bhatia, R. Bhatia, and G. P. Dubey, Phys. Chem. Liq. 10, 1080 (2009).
S. C. Bhatia, R. Bhatia, and G. P. Dubey, J. Chem. Thermodyn. 41, 1132 (2009).
G. P. Dubey, M. Sharma, and N. Dubey, J. Chem. Thermodyn. 40, 309 (2008).
R. Meyer, M. Meyer, J. Metzger, and A. Peneloux, J. Chim. Phys. Phys. Chim. Biol. 63, 406 (1971).
K. Rajagopal and S. Chenthilnath, J. Mol. Liq. 160, 72 (2011).
M. Karvo, J. Chem. Thermodyn. 18, 809 (1986).
H. I. Khani, N. Z. Orasna, and R. S. Imani, J. Phys. Chem. Liq. 43, 391 (2005).
B. R. Kumar, P. M. Krishna, S. A. Banu, K. A. Jyothi, T. S. Jyostna, and N. Sathyanarayana, Phys. Chem. Liq. 48, 79 (2010).
P. Venkateswarlu, K. Rambabu, N. V. Chowdary, and G. K. Raman, Indian J. Technol. 28, 27 (1990).
G. N. Swami, G. D. Raju, and G. K. Raman, Can. J. Chem. 59, 229 (1980).
ACKNOWLEDGMENTS
Authors are thankful to university grants commission (UGC), Government of India, for financial support in the form of UGC-JRF.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Savitha Jyostna, T., Satheesh, B., Sreenu, D. et al. Physical-Chemical Properties of Binary Liquid Mixtures of Isoamyl Alcohol with Chloroethanes at 298–308 K. Russ. J. Phys. Chem. 93, 278–287 (2019). https://doi.org/10.1134/S0036024419020249
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419020249