Abstract
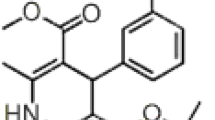
A selective, precise and new high-performance liquid chromatographic method for the analysis of loperamid hydrochloride in pharmaceutical formulations was developed and validated. The mobile phase consisting buffer (sodium-octansulphonate, triethylamine and ammonium hydroxide) in water: acetonitriie (45: 55, v/v) (pH 3.2). The absorbance was monitored with a DAD detector at 226 nm. The flow rate was 1.5 cm3 min−1. The linearity (r = 0.9947) and the recovery (98.58–100.42%) were found to be satisfactory. The detection and quantitation limits were found to be 0.95 and 3.12 μg cm−3. The results demonstrated that the procedure was accurate, precise and reproducible. It can be suitably applied for the estimation of lopera-mid hydrochloride in pharmaceutical formulations.
Similar content being viewed by others
References
K. Lavrijsen, D. Van Dyck, J. Van Houdt, J. Hendrickx, J. Monbaliu, R. Woestenborghs, W. Meuldermans, and J. Heykants, J. Pharmacol. Exp. Ther. 23, 354 (1995).
D. L. DeHaven-Hudkins, L. Cortes Burgos, J. A. Cassel, J. D. Daubert, R. N. DeHaven, E. Mansson, H. Nagasaka, G. Yu, and T. Yaksh, J. Pharmacol. Exp. Ther. 289, 494 (1999).
The European Agency for the Evaluation of Medicinal Products, ICH Topic Q2B Note for Guideline on Validation of Analytical Procedures: Methodology, GPMP/ICH/281/95 (1996).
Jugoslovenska Pharmacopoeia, 5th ed. (Savrem. administracija, Beograd, 2000).
S. Bolton, Pharmaceutical Statistics: Practical and Clinical Application, 3rd ed. (Marcel Dekker, New York, 1997), pp. 216–264.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Nikolić, G.S., Savić, I. & Marinković, V. Development and validation of a new high-performance liquid chromatographic method for the loperamid hydrochloride determination in drugs. Russ. J. Phys. Chem. 83, 1609–1611 (2009). https://doi.org/10.1134/S0036024409090349
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024409090349