Abstract
Interaction of zinc(II)- and copper(II) perchlorate hexahydrates with nicotinamide (Nia – nicotinamide, niacinamide, 3-pyridinecarboxamide, C5H4NС(O)NH2) has been studied. It has been demonstrated that complex compounds [Zn(Nia)2(H2O)4](ClO4)2 (1) and [Cu(Nia)2(H2O)2](ClO4)2 ⋅ 2H2O (2) are formed in aqueous media at the molar ratio M(ClO4)2 ⋅ 6H2O : Nia = 1 : 2. Both compounds are the ionic ones. Geometry of complex cation (1) may be represented as a distorted octahedron in which nicotinamide molecules are in the trans-position. The same position of ligands is found for planar complex cation (2). Cytotoxicity of the prepared compounds (MTT assay) has been determined with respect to dental pulp stem cells (DPSC) and breast cancer cell line MCF-7. Antiproliferative activity has been studied relative to 10 cancer cell lines, complex compound (1) being the most toxic for C6, Panc-1, U251 cell lines (survivability below 15%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
At the present time cancer, with the exception of COVID-19 and cardiovascular diseases, is a leading cause of death worldwide [1]. Platinum-based drugs (cisplatin, oxaliplatin, carboplatin, etc.) are widely used for chemotherapy of various origin tumors. Their action is based on the formation with the DNA (deoxyribonucleic acid) inter- and intra-helical chemical bonds via N7 of purine bases, which results to the replication and transcription disrupting, apoptosis, and discontinuation of cellular proliferation and tumor growth. The mentioned mechanism is the non-specific one and platinum-containing compounds demonstrate serious side effects (neuro-, hepato-, and nephrotoxicity). That is why complex compounds of transition metals, rare-earth elements with small organic ligands as potential anticancer pharmacological agents of new generation are of special interest [2–9]. Action of metal-complexes-containing anticancer drugs is based on the different adducts formation with DNA [10]. In this relation, intercalation is considered as one of the most important modes of non-covalent interaction of bioactive species with DNA (excluding electrostatic interactions and binding in minor grooves) [10, 11].
Cyclin-dependent kinases, proteins and aminoacids, and more complicated ones such as singular organelles—mitochondria, are another target in the cell. A number of organic compounds used in pharmaceutics are activated or bio transformed by metal ions, for example, by copper- and zinc-cations, which are involved in various cellular processes and demonstrate complex action on a cell [12].
Copper and zinc are essential elements for living organisms; they promote different biochemical reactions and capable to show an anticancer activity. Copper-containing species are cofactors of a number of enzymes, for example, superoxide dismutase, neutralizing free oxygen radicals. They demonstrate as well selective cytotoxicity with respect to cancer cells, due to reduced oxygen content in the area surrounding malignant tumor, resulting to reduction of Cu(II) in the depleted in oxygen tumor cells to Cu(I) catalyzing formation of reactive oxygen species, oxidative stress, the DNA double helix rupture and apoptosis.
The role of copper ions as proteasome inhibitors has been described in [13], possibly they can stimulate autophagy in cells [14]. It is known that copper atoms not only are constituents of anticancer agents, but also can enhance their action [15].
Zinc is one of the most important essential nutrients playing role of a regulating ion in cells [16]. Except that, zinc is a constituent of numerous enzymes with anticancer action.
Nicotinamide (Nia, amide of nicotinic acid)— vitamin B3, is a precursor of nicotinamide-adenine dinucleotide. It is known that nicotinamide possesses definite antitumor properties and may be efficient in cancer chemoprevention and therapy [17] against pre-cancer skin state (actinic keratosis) and cutaneous squamous cell carcinoma—the second in incidence skin cancer [18]. It has been demonstrated that intake of aminoacids and vitamins facilitates the drug selectivity [13].
Data on platinum(II)- and silver(I) complex compounds with nicotinamide are given for cis-[Pt(Nia)(NH3)2Cl]NO3 [19] and [Ag(Nia)2]NO3·H2O [20]. It has been found that [Ag(Nia)2]NO3·H2O demonstrates higher cytotoxic activity with respect to a mouse lymphocytic leukemia cell line L1210 (IC50 = 1.23 ± 0.22 μM) in comparison with cisplatin (IC50 = 3.40 ± 0.20 μM) [20].
The structure of the zinc(II) nitrate complex compound with nicotinamide [Zn(Nia)2(H2O)4](NO3)2⋅ 2H2O, prepared from zinc(II) nitrate and nicotinamide in ethanolic solution, has been investigated in [21], but its bioactivity has not been studied. Synthesis of copper(II) perchlorate complex with nicotinamide [Cu(Nia)6](ClO4)2 in acetonitrile solution has been described; complex cation of this compound represents octahedron, where coordinated (via pyridine ring nitrogen atoms) nicotinamide molecules are located in the octahedron vertices [22].
To the best of our knowledge, prepared from aqueous solutions zinc(II)- and copper(II) perchlorate complexes with nicotinamide were not studied. Therefore the aim of the present work consists in synthesis and studies on structure and properties of zinc(II)- and copper(II) perchlorate complexes with nicotoneamide and in comparison of their bioactivity.
EXPERIMENTAL
Materials. Zinc(II) and copper(II) perchlorate hexahydrates were preliminary prepared from the respective basic carbonates 3Zn(OH)2⋅2ZnCO3 (99%, Reachim), Cu(OH)2⋅CuCO3 (99%, ABCR) and perchloric acid (ch., Reachim). Nicotinamide (99.5%, Sigma-Aldrich) was used for synthesis.
Methods
Contents of chemical elements were determined using the CHNS EuroVector EuroEA 3000 (EuroVector s.p.a., Italy) elemental analyzer. Metal content was determined by trilonometric titration and using the inductively coupled plasma atomic emission spectroscopy (ICP-AES) iCAP 6300 DUO (Thermo Scientific, USA) in the Shared Knowledge Center NRC “Kurchatov Institute”—IREA.
IR-spectra were recorded at the Bruker Vertex 70 FTIR spectrometer with Ram II Raman attachment in the area 350–4000 cm–1 in tablets with KBr.
Electrospray ionization mass spectra (ESI-MS) were obtained on an AmaZon Bruker Daltonik GmbH mass spectrometer (acquired over the mass range m/z = 70–2200) in positive and negative ion modes in a H2O–MeCN solvent mixture (1 : 1).
Powder X-ray diffraction (pXRD) patterns were performed on a Bruker D8 Advance diffractometer (CuKα radiation, Ni-filter, LYNXEYE detector, reflection geometry) over the 2θ range from 5° to 80° and the step of 0.01125°) in the Shared Equipment Centre of the Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences.
Single-crystal X-ray diffraction data for compounds 1 and 2 were collected on a CCD area Bruker D8 Venture diffractometer (graphite monochromator, MoKα radiation, ω-scanning technique, 150 K). The X-ray diffraction data were processed using the SAINT program [23]. An absorption correction was applied with the SADABS program. The crystal structure was solved by direct methods and refined based on F2 by the full-matrix least-squares method with anisotropic displacement parameters for nonhydrogen atoms. The hydrogen atoms were positioned geometrically and refined by the least-squares method using a “riding” model. All calculations were carried out with the Olex-2 software [24] and SHELXTL-Plus program package. [25]. The crystal structure visualization was performed using the MERCURY program. [26]. The structural data were deposited with the Cambridge Crystallographic Data Centre (CCDC 2121923 (1) and 2121926 (2)) and are available, free of charge, at http://www.ccdc.cam.ac.uk.
Cytotoxicity of the prepared compounds was studied using the MTT colorimetric assay for 24 h in postnatal human dental pulp stem cells (DPSC) and the MCF-7 cell line (breast cancer cell line) obtained from the Russian Collection of Cell Cultures (Institute of Cytology of the Russian Academy of Sciences) [27, 28]. The statistical data processing was performed using the Origin program, the error was taken as the root-mean-square deviation from the mean values, and the statistically significant difference was determined using the Mann–Whitney U test at р < 0.01.
Antiproliferative activity was studied with respect to ten different cancer cell lines: a rat glioma (C6), a human pancreatic cancer cell line (Panc-1), a human glioblastoma (U251), a human neuroblastoma (IMR32), a human neuroblast-derived SH-SY5Y cell line, breast cancer cell line (HS 578T and BT474), human embryonic kidney cells (HEK293), laryngeal cancer cells (Hep-2) and osteosarcoma MNNG/HOS cells, at the compound concentration с = 1 × 10–4 mol L–1.
Experiment. Copper(II) and zinc(II) perchlorate hexahydrates were obtained by a reaction of copper(II)- and zinc(II) basic carbonates Cu(OH)2⋅ CuCO3 and 3Zn(OH)2⋅2ZnCO3, respectively, with perchloric acid taken in the molar ratio carbonate : HClO4 = 1 : 2. The prepared solutions were heated to evaporate 60–70% water and then were cooled to room temperature. Zinc(II) perchlorate hexahydrate was crystallized as colorless prisms, while copper(II) perchlorate hexahydrate—as blue prisms. The compound compositions are consistent with M(ClO4)2⋅ 6H2O, (M = Cu, Zn). The sample homogeneity was confirmed by powder XRD.
Zinc(II)- and copper(II) perchlorate complexes with nicotinamide [Zn(Nia)2(H2O)4](ClO4)2 (1) and [Cu(Nia)2(H2O)2](ClO4)2 ⋅ 2H2O (2) were prepared by a reaction of the preliminary obtained zinc(II) perchlorate hexahydrate (1.8619 g, 5 mmol) and copper(II) perchlorate hexahydrate (1.8527 g, 5 mmol) dissolved in water (10 mL) with aqueous solution (10 mL) of nicotinamide (1.2213 g, 10 mmol) at the molar ratio M(ClO4)2 : Nia = 1 : 2 (M = Zn, Cu) (scheme S1, S2). The mentioned molar ratios of components were used on the basis of results given in [21]. The combined solution color was changed from colorless to pale yellow for (1) and from blue to dark blue for (2). The prepared compounds were filtered off using filtering funnel with porous bottom, washed with a minimal volume of water and dried in a desiccator over sodium hydroxide. Yield: 80–85%.
Tetraaquabis(nicotinamide)zinc(II) perchlorate [Zn(Nia)2(H2O)4](ClO4)2 (1). Yield 2.32 g, 80%.
For C12H20Cl2N4O14Zn (1) (580.58) anal. calcd., wt %: C, 24.83; N, 9.65; H, 3.47; Zn, 11.26. Found wt %: C, 24.58; N, 9.47; H, 3.67; Zn, 11.07.
IR-spectra (cm–1) 827 ρ(H2O); 1070 ν3(ClO4–); 1430 ν(Zn–N) + δ(CNC); 1608 δ(H2O); 1640 ν(С=O); 3350–3450 ν(N–H) (Table S1).
ESI-MS spectrum (4.5 kV, m/z (Irel, %)), found/calc.: 225.97/218.89 [Zn(H2O)3(ClO4)]+ (7.7), 256.99/254.92 [Zn(H2O)5(ClO4)]+ (10.8), 329.01/322.99 [Zn(Nia)(H2O)2(ClO4)]+ (13.1), 358.93/359.02 [Zn(Nia)(H2O)4(ClO4)]+ (35.6), 406.97/409.09 [Zn(Nia)2(ClO4)]+ (100), 479.01/479.31 [Zn(Nia)2(H2O)4(ClO4)]+ (13.5), 531.00/531.221 [Zn(Nia)3(ClO4)]+ (21.4); 99.04/99.45 (ClO4)– (3.4), 362.55/363.74 [Zn(ClO4)3]– (100), 486.61/485.87 [Zn(Nia)(ClO4)3]– (5.0); and nicotinamidium cation 123.28/123.13 [NiaH]+ (14.6) (Figs. S1a, S1b).
Diaquabis(nicotinamide)copper(II) perchlorate dihydrate [Cu(Nia)2(H2O)2](ClO4)2⋅2H2O (2). Yield 2.45 g, 84.7%.
For C12H20Cl2N4O14Cu (2) (578.76) anal. calcd., wt %: C, 24.90; N, 9.68; H, 3.48; Cu, 10.98. Found wt %: C, 24.27; N, 9.97; H, 3.65; Cu, 11.00.
IR-spectra (cm–1) 837 ρ(H2O) ; 1060 ν3\({\text{(ClO}}_{4}^{ - })\); 1440 ν(Cu–N) + δ(CNC); 1603 δ(H2O); 1670 ν(С=O); 3100–3400 ν(N–H); 3600 ν(O–H) (Table S1).
ESI-MS spectrum (4.5 kV, m/z (Irel, %)), found/calc.: 225.94/217.04 [Cu(H2O)3(ClO4)]+ (19.8), 326.85/321.15 [Cu(Nia)(H2O)2(ClO4)]+ (10.2), 355.91/357.18 [Cu(Nia)(H2O)4(ClO4)]+ (13.3), 405.91/407.16 [Cu(Nia)2(ClO4)]+ (100); 98.98/99.45 (ClO4)– (3.6), 361.49/361.9 [Cu(ClO4)3]– (100), 483.58/484.03 [Cu(Nia)(ClO4)3]– (13.2), 605.71/606.15 [Cu(Nia)2(ClO4)3]– (8.7); and nicotinamidium cation 123.20/123.13 [NiaH]+ (3.8) (Figs. S1c, S1d).
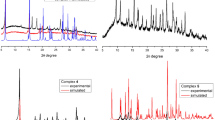
Single crystals of compound 1 were grown as light yellow prisms after isothermal (room temperature) solvent evaporation for 3-4 d. Single crystals of compound 2 as dark blue blocks were obtained next day after synthesis. Identity of the prepared samples was confirmed by comparison of the pXRD patterns for reagents, target products and with the theoretical pXRD patterns (from the respective single crystal data) as well (Fig. S2). Therefore, the prepared compounds were isolated as the individual ones without impurities of reagents or other substances and diffraction patterns calculated from the single crystal data are compatible with the experimental ones, that is why the single crystal structures for 1 and 2 are representatives of the respective sample in the bulk.
RESULTS AND DISCUSSION
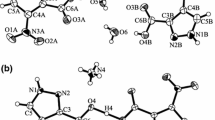
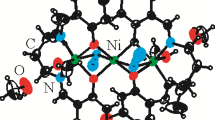
According to the single crystal XRD data compounds 1 and 2 are the ionic ones. In complex cation 1 zinc(II) is located in the center of slightly distorted octahedron formed by nitrogen atoms of two trans-nicotinamide molecules and oxygen atoms of four water molecules (Fig. 1a), while planar complex cation 2 is formed by nitrogen atoms of two trans-nicotinamide molecules and oxygen atoms of two water molecules (Fig. 1b). Crystal structure data and the main bond lengths and angles for compounds are given in Tables S2, and S3, S4.
Comparison M–O and M–N bond lengths demonstrates that for [Cu(Nia)2(H2O)2](ClO4)2⋅2H2O (2) the Cu–O and Cu–N bond lengths are equal to 1.9658(10)–1.9800(9) and 1.989(1)–2.005(1) Å, respectively. For compound (1) the Zn–O and Zn–N bond lengths are equal to 2.101(1)–2.104(1) and 2.155(1) Å, respectively (Tables S3, S4) and are compatible with those for [Zn(Nia)2(H2O)4](NO3)2·2H2O [21]. This fact is determined by changes in the complex cation size with coordination number growth. Both compounds are characterized by the presence of H-bonding with participation of perchlorate-ions, amide group of nicotinamide and coordinated and non-coordinated water molecules. Unlike copper compound (2), complex compound (1) is additionally stabilized due to π–π interaction between pyridine fragments of nicotinamide molecules (angle between planes = 0°, distance between ring centroids: 2.325 Å).
As can be seen from the data given in Tables S5 and S6 and Fig. S3, all samples demonstrate dose dependent behavior on cells, compound (1) being more toxic for DPSC at c = 5 × 10–5–1 × 10–4 mol L–1 than for the respective stoichiometric mixtures. For lower concentrations, there is not reliable difference between samples and respective mixtures, which may be explained by relatively higher toxicity of perchlorate ions. However, for compound 1 (с = 1 × 10–5 mol L–1) DPSC survivability is higher (in comparison with MCF-7) and equals to 95.40 ± 9.25 (MCF-7 survivability: 89.21 ± 18.08%). At the same time compound 1 suppresses survivability of MCF-7 cell line (in comparison with zinc(II) perchlorate and its stoichiometric mixture with nicotinamide) and approaches the toxic action of doxorubicin at the same concentration (89.21 ± 18.08 and 81.73 ± 9.23% respectively, .).
It has been demonstrated [29] that zinc influence on apoptosis depends on a number of factors, primarily on cell type: low zinc concentrations induce apoptosis of certain cells, while action of high zinc concentrations inhibits apoptosis. Furthermore, zinc is able to regulate proliferation of cells and their growth [29]. In certain cases, zinc treatment initiates p53/ROS-mediated apoptotic events, which include translocation of p53 to mitochondria, dissipation of mitochondrial membrane potential, and direct mitochondrial translocation of pro-apoptotic gene Bax. In was found that zinc-induced apoptosis in MCF-7 breast cancer cells requires the presence of a functional p53 expression [29].
As can be seen from Fig. 2, zinc(II) perchlorate complex with nicotinamide demonstrates strong antiprofilerative action on cells (survivability, % with respect to control, c = 1 × 10–4 mol L–1): C6 (6.02 ± 0.96%), Panc-1 (12.41 ± 1.97%), U251 (7.05 ± 1.98%), SH-Sy-5Y (30.00 ± 5.72%), HS578T (19.66 ± 5.58%), HEK293 (15.16 ± 2.07%), IMR32 (33.80 ± 2.89%), as well as moderate effect on Hep-2 (60.63 ± 11.62%), BT474 (47.86 ± 5.15%).
It turned out, that MNNG-HOS cell line is the most resistant to [Zn(Nia)2(H2O)4](ClO4)2 (1) (survivability: 96.49 ± 6.03%) in comparison with DPSC (survivability: 67.91 ± 5.67%). The copper(II) perchlorate complex with nicotinamide (2) hardly shows anticancer action at the same concentration, the latter being possibly related with lower stability of [Cu(Nia)(H2O)2(ClO4)]+ in comparison with [Zn(Nia)(H2O)4(ClO4)]+ in aqueous medium. It is confirmed by the relative intensities (Irel, %) of the respective signals in ESI-MS spectra: 10.2 for [Cu(Nia)(H2O)2(ClO4)]+ and 35.6 for [Zn(Nia)(H2O)4(ClO4)]+. But cytotoxic effect of (1) on C6, Panc-1 and U251 cell lines is significant (survivability below 15%).
CONCLUSIONS
Complex compounds [Zn(Nia)2(H2O)4](ClO4)2 (1) and [Cu(Nia)2(H2O)2](ClO4)2⋅2H2O (2) have been prepared and identified for the first time, their structure and properties have been investigated. Comparative studies on cytotoxicity of [Zn(Nia)2(H2O)4](ClO4)2 (1) and [Cu(Nia)2(H2O)2](ClO4)2⋅2H2O (2) have been performed with respect to stem and cancer cells. Dose-dependent cytotoxicity values have been determined for all types of cells. The efficacy of the zinc-containing compound (1) action on C6, Panc-1, U251 cell lines (survivability below 15%) has been demonstrated on the basis of the comparative studies on the compound antiproliferative activity with respect to 10 cell lines.
The results of the present work are in accordance with the literature data on bioactivity of zinc(II)- and copper(II) complex compounds with pyrazole derivatives, phosphor-containing analogues of salicylic acid, and other ligands containing N- and O-donor atoms [30–32]. Additional investigations are needed for studies on the compound bioactivity both in vitro and in vivo.
REFERENCES
Cancer Research in UK. Worldwide Statistics. www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer/incidence/heading-One.
I. Kostova, Recent Patents on Anti-Cancer Drug Discovery 1, 1 (2006). https://doi.org/10.2174/157489206775246458
N. S. Rukk, L. G. Kuzmina, D. V. Albov, et al., Polyhedron 102, 152 (2015). https://doi.org/10.1016/j.poly.2015.09.011
L. Findoráková, K. Győryová, D. Hudecová, et al., J. Therm. Anal. Calorim. 111, 1771 (2006). https://doi.org/10.1007/s10973-012-2275-9
N. P. E. Barry and P. J. Sadler, Chem. Commun. 49, 5106 (2013). https://doi.org/10.1039/c3cc41143e
N. S. Rukk, D. V. Albov, R. S. Shamsiev, et al., Polyhedron 44, 124 (2012). https://doi.org/10.1016/j.poly.2012.06.075
N. S. Rukk, L. G. Kuz’mina, G. A. Davydova, et al., Izv. AN. Ser. Khim. 7, 1394 (2020).
N. S. Rukk, L. G. Kuzmina, R. S. Shamsiev, et al., Inorg. Chim. Acta 487, 184 (2019). https://doi.org/10.1016/j.ica.2018.11.036
I. S. Golubeva, N. P. Yavorskaya, M. A. Baryshnikova, et al., Ros. Bioter. Zh. 15, 89 (2016). https://doi.org/10.17650/1726-9784-2016-15-4-89-95
R. D. Boer, A. Canals, and M. Coll, Dalton Trans. 3, 399 (2009). https://doi.org/10.1039/b809873p
L. H. Hurley, Nat. Rev. Canc. 2, 188 (2002). https://doi.org/10.1038/nrc749
M. Marloye, G. Berger, M. Gelbcke, et al., Future Med. Chem. 8, 2263 (2016). https://doi.org/10.4155/fmc-2016-0153
Zhen Zhang, Huiyun Wang, Maocai Yan et al., Mol. Med. Rep. 15, 3 (2017). https://doi.org/10.3892/mmr.2016.6022
C. Molinaro, A. Martoriati, L. Pelinski, et al., Cancers 12, 2863 (2020). https://doi.org/10.3390/cancers12102863
H. Mizutani, A. Nishimoto, S. Hotta, et al., Anticancer Res. 38, 2643 (2018). https://doi.org/10.21873/anticanres.12506
C. T. Chasapis, P.-S. A. Ntoupa, C. A. Spiliopoulou, et al., Arch. Toxicol. 94, 1443 (2020). https://doi.org/10.1007/s00204-020-02702-9
I. P. Nikas and S. A. Paschou, and Han Suk Ryu, Biomolecules 20, 477 (2020). https://doi.org/10.3390/biom10030477
L. Fania, C. Mazzanti, E. Campione, et al., Int. J. Mol. Sci. 20, 5946 (2019). https://doi.org/10.3390/ijms20235946
B. Wang, H. Qian, S. -M. Yiu, et al., Eur. J. Med. Chem. 71, 366 (2014). https://doi.org/10.1016/j.ejmech.2013.10.062
M. Rendosova, Z. Vargova, J. Kuchar, et al., J. Inorg. Biochem. 168, 1 (2017). https://doi.org/10.1016/j.jinorgbio.2016.12.003
A. Dziewulska-Kulaczkowska, L. Mazur, and W. Ferenc, J. Therm. Anal. Calorim. 96, 255 (2009). https://doi.org/10.1007/s10973-008-9851-z
K.-L. H. Chen and R. T. Iwamoto, Inorg. Chim. Acta 3, 223 (1969). https://doi.org/10.1016/s0020-1693(00)92483-6
Bruker 2001. SAINT (Version 6.02a). Bruker AXS Inc., Madison, Wisconsin, USA.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009). https://doi.org/10.1107/S0021889808042726
SHELXTL-Plus, Version 5.10, Bruker AXS Inc., Madison, Wisconsin, USA, 1997.
C. F. Macrae, I. J. Bruno, J. A. Chisholm, et al., J. Appl. Crystallogr. 41, 466 (2008). https://doi.org/10.1107/S0021889807067908
T. Mossman, J. Immunol. Methods 65, 55 (1983).
R. A. Poltavtseva, Yu. A. Nikonova, I. I. Selezneva, et al., Bull. Exp. Biol. Med. 158, 164 (2014). https://doi.org/10.1007/s10517-014-2714-7
R. B. Franklin and L. C. Costello, J. Cellular Biochem. 106, 750 (2009). https://doi.org/10.1002/jcb.22049
A. D. Ivanova, T. A. Kuz’menko, A. I. Smolentsev, et al., Russ. J. Coord. Chem. 47, 751 (2021). https://doi.org/10.1134/S1070328421110026
I. S. Ivanova, G. S. Tsebrikova, Yu. I. Rogacheva, et al., Russ. J. Inorg. Chem. 66, 1846 (2021). https://doi.org/10.1134/S0036023621120068
P. Marinova, M. Marinov, M. Kazakova, et al., Russ. J. Inorg. Chem. 66, 1925 (2021). https://doi.org/10.1134/S0036023621130052
ACKNOWLEDGMENTS
Analytical studies, IR spectroscopy, ESI-MS spectro-metry were performed using equipment of NRC “Kurchatov Institute”—IREA Shared Knowledge Center under project’s financial support by the Russian Federation, represented by The Ministry of Science and Higher Education of the Russian Federation, Agreement no. 075-11-2021-070 dd. 19.08.2021.
X-ray diffraction studies were performed at the User Facilities Center of IGIC RAS within the State Assignment on Fundamental Scientific Researches to the Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences and with the financial support of the Council for Grants of the President of the Russian Federation MK-5992.2021.1.3. Studies in vitro were performed within the State Assignment on Fundamental Scientific Researches to the Institute of Theoretical and Experimental Biophysics of the Russian Academy of Sciences 075-00381-21-00.
Funding
The present work was performed with the financial support of the Council for Grants of the President of the Russian Federation MK-5992.2021.1.3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ADDITIONAL INFORMATION
The article was prepared on the basis of materials of the XXVIII International Chugaev Conference on Coordination Chemistry (Ol’ginka, Tuapse oblast, Russia, October 3–8, 2022).
Additional information
Translated by N. Rukk
Supplementary Information
Rights and permissions
About this article
Cite this article
Rukk, N.S., Kabernik, N.S., Buzanov, G.A. et al. Complexes of Zinc(II)- and Copper(II) Perchlorates with Nicotinamide: Synthesis, Structure, Cytotoxicity. Russ. J. Inorg. Chem. 67, 1184–1190 (2022). https://doi.org/10.1134/S0036023622080228
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622080228