Abstract
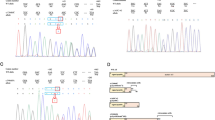
Antithrombin III (AT3) belongs to the superfamily of serine protease inhibitors (serpins) and is a major anticoagulant in physiological conditions. Based on SERPINC1 gene, a minigene coding for human AT3, which is valuable for medicine and biotechnology, was constructed by minimizing the size of lengthy introns and preserving the splicing site-flanking sequences. An analysis of the minigene splicing pattern identified one correct AT3 transcript and two alternatively spliced transcripts, which formed either due to minigene exons 2 and 3 skipping or an aberrant exon insertion via splicing at cryptic splicing sites in intron 1 of the minigene. Site-directed mutagenesis of the cryptic splicing sites successfully optimized the splicing pattern of the AT3 minigene to completely prevent the generation of the alternative transcripts. The presence of the cryptic splicing sites in intron 1 of the minigene was confirmed with Human Splicing Finder v. 3.1 software, thus demonstrating that putative alternative splicing sites are possible to identify in minimized or hybrid introns of minigenes and to eliminate via mutagenesis before experimentally testing the minigene splicing patterns. The approach to the design of minigenes together with the bioinformatical analysis of the nucleotide sequences of minigene introns can be used to construct minigenes in order to generate transgenic animals producing economically valuable proteins in the milk.


Similar content being viewed by others
REFERENCES
Luxembourg B., Delev D., Geisen C., Spannagl M., Krause M., Miesbach W., Heller C., Bergmann F., Schmeink U., Grossmann R., Lindhoff-Last E., Seifried E., Oldenburg J., Pavlova A. 2011. Molecular basis of antithrombin deficiency. Thromb. Haemost. 105, 635–646.
Konkle B.A., Bauer K.A., Weinstein R., Greist A., Holmes H.E., Bonfiglio J. 2003. Use of recombinant human antithrombin in patients with congenital antithrombin deficiency undergoing surgical procedures. Transfusion. 43, 390–394.
Paidas M.J., Triche E.W., James A.H., DeSancho M., Robinson C., Lazarchick J., Ornaghi S., Frieling J. 2016. Recombinant human antithrombin in pregnant patients with hereditary antithrombin deficiency: Integrated analysis of clinical data. Am. J. Perinatol. 33, 343–349.
Shepelev M.V., Kalinichenko S.V., Deykin A.V., Korobko I.V. 2018. Production of recombinant proteins in the milk of transgenic animals: Current state and prospects. Acta Naturae. 10, 40–47.
Lavine G. 2009. FDA approves first biological product derived from transgenic animal. Am. J. Health Syst. Pharm. 66 (6), 518. https://doi.org/10.2146/news090023
Edmunds T., Van Patten S.M., Pollock J., Hanson E., Bernasconi R., Higgins E., Manavalan P., Ziomek C., Meade H., McPherson J.M., Cole E.S. 1998. Transgenically produced human antithrombin: Structural and functional comparison to human plasma-derived antithrombin. Blood. 91, 4561–4571.
Palmiter R.D., Sandgren E.P., Avarbock M.R., Allen D.D., Brinster R.L. 1991. Heterologous introns can enhance expression of transgenes in mice. Proc. Natl. Acad. Sci. U. S. A. 88, 478–482.
Choi T., Huang M., Gorman C., Jaenisch R. 1991. A generic intron increases gene expression in transgenic mice. Mol. Cell. Biol. 11, 3070–3074.
Raker V.A., Mironov A.A., Gelfand M.S., Pervouchine D.D. 2009. Modulation of alternative splicing by long-range RNA structures in Drosophila. Nucleic Acids Res. 37, 4533–4544.
Gelfand M.S. 1989. Statistical analysis of mammalian pre-mRNA splicing sites. Nucleic Acids Res. 17, 6369–6382.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by T. Tkacheva
Abbreviations: AT3, antithrombin III; UTR, untranslated region.
Rights and permissions
About this article
Cite this article
Shepelev, M.V., Saakian, E.K., Kalinichenko, S.V. et al. Human Antithrombin III Minigene with an Optimized Splicing Pattern. Mol Biol 53, 362–370 (2019). https://doi.org/10.1134/S0026893319030178
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893319030178