Abstract
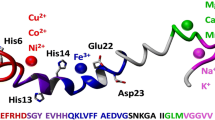
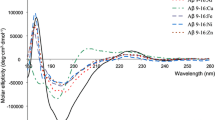
Complexes of peptide fragment 1–16 of beta-amyloid with transition metals play an important role in the development of a broad class of neurodegenerative diseases, which determines the interest in investigating the structures of these complexes. In this work, we have applied the method of the deuterium/hydrogen exchange in combination with ultra-high-resolution mass spectrometry to study conformational changes in (1–16) beta-amyloid peptide induced by binding of zinc(II) atoms. The efficiency of the deuterium/hydrogen exchange depended on the number of zinc atoms bound to the peptide and on the temperature of the ionization source region. Deuterium/hydrogen exchange reactions have been performed directly in the ionization source. The number of exchanges decreased considerably with an increasing numbers of zinc atoms. The relationship has been described with a damped exponential curve, which indicated that the binding of zinc atoms altered the conformation of the peptide ion by making it less open, which limits the access to inner areas of the molecule.
Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- Aβ:
-
beta-amyloid
- ICR:
-
ion cyclotron resonance
References
Zawisza I., Rozga M., Bal W. 2012. Affinity of copper and zinc ions to proteins and peptides related to neurodegenerative conditions (A beta, APP, alpha-synuclein, PrP). Coord. Chem. Rev. 256, 2297–2307.
Taraszka J.A., Li J.W., Clemmer D.E. 2000. Metalmediated peptide ion conformations in the gas phase. J. Phys.Chem. B. 104 (18), 4545–4551.
Viles J.H. 2012. Metal ions and amyloid fiber formation in neurodegenerative diseases. Copper, zinc and iron in Alzheimer’s, Parkinson’s and prion diseases. Coord. Chem. Rev. 256, 2271–2284.
Kozlowski H., Luczkowski M., Remelli M., Valensin D. 2012. Copper, zinc and iron in neurodegenerative diseases (Alzheimer’s, Parkinson’s and prion diseases). Coord. Chem. Rev. 256, 2129–2141.
Miller Y., Ma B.Y., Nussinov R. 2012. Metal binding sites in amyloid oligomers: Complexes and mechanisms. Coord. Chem. Rev. 256, 2245–2252.
Kulikova A.A., Makarov A.A., Kozin S.A. 2015. Roles of zinc ions and structural polymorphism of ß-amyloid in the development of Alzheimer’s disease. Mol. Biol. (Moscow). 49 (2), 217–230.
Carlton D.D., Schug K.A. 2011. A review on the interrogation of peptide–metal interactions using electrospray ionization-mass spectrometry. Anal. Chimica Acta. 686, 19–39.
Popov I.A., Indeikina M.I., Pekov S.I., Starodubtseva N.L., Kononikhin A.S., Nikolaeva M.I., Kukaev E.N., Kostyukevich Yu.I., Kozin S.A., Makarov A.A., Nikolaev E.N. 2014. Estimation of phosphorylation level of amyloid-beta isolated from human blood plasma: Ultrahigh-resolution mass spectrometry Mol. Biol. (Moscow). 48 (4), 607–614.
Kostyukevich Y., Kononikhin A., Popov I., Indeykina M., Kozin S.A., Makarov A.A., Nikolaev E. 2015. Supermetallization of peptides and proteins during electrospray ionization. J. Mass Spectrom. 50 (9), 1079–1087.
Kostyukevich Y., Kononikhin A., Popov I., Kukaev E., Shiea J., Nikolaev E. 2016. Supermetallization of peptides and proteins with tetravalent metal Th(IV). Eur. J. Mass Spectrom. (Chichester). 22 (1), 39–42.
Kostyukevich Y.I., Kononikhin A.S., Popov I.A., Indeykina M.I., Nikolaev E.N. 2016. Supermetallization of substance P during electrospray ionization. Mendeleev Commun. 26 (2), 111–113.
Kulikova A.A., Tsvetkov P.O., Indeykina M.I., Popov I.A., Zhokhov S.S., Golovin A.V., Polshakov V.I., Kozin S.A., Nudler E., Makarov A.A. 2014. Phosphorylation of Ser8 promotes zinc-induced dimerization of the amyloidbeta metal-binding domain. Mol. Biosystems. 10 (10), 2590–2596.
Kozin S.A., Mezentsev Y.V., Kulikova A.A., Indeykina M.I., Golovin A.V., Ivanov A.S., Tsvetkov P.O., Makarov A.A. 2011. Zinc-induced dimerization of the amyloid-beta metal-binding domain 1–16 is mediated by residues 11-14. Mol. Biosystems. 7 (4), 1053–1055.
Khmeleva S.A., Mezentsev Yu.V., Kozin S.A., Mitkevich V.A., Medvedev A.E., Ivanov A.S., Bodoev N.V., Makarov A.A., Radko S.P. 2015. Effect of mutations and modifications of amino acid residues on zincinduced interaction of the metal-binding domain of ß-amyloid with DNA. Mol. Biol. (Moscow). 49 (3), 450–456.
Katta V., Chait B.T. 1993. Hydrogen-deuterium exchange electrospray-ionization mass-spectrometry: A method for probing protein conformational-changes in solution. J. Am. Chem. Soc. 115 (14), 6317–6321.
Wales T.E., Engen J.R. 2006. Hydrogen exchange mass spectrometry for the analysis of protein dynamics. Mass Spectrom. Rev. 25 (1), 158–170.
Gard E., Green M.K., Bregar J., Lebrilla C.B. 1994. Gas-phase hydrogen-deuterium exchange as a molecular probe for the interaction of methanol and protonated peptides. J. Am. Soc. Mass Spectrom. 5 (7), 623–631.
McLafferty F.W., Guan Z.Q., Haupts U., Wood T.D., Kelleher N.L. 1998. Gaseous conformational structures of cytochrome c. J. Am. Chem. Soc. 120 (19), 4732–4740.
Freitas M.A., Hendrickson C.L., Emmett M.R., Marshall A.G. 1999). Gas-phase bovine ubiquitin cation conformations resolved by gas-phase hydrogen/deuterium exchange rate and extent. Int. J. Mass Spectrom. 185, 565–575.
Price N. P. J. 2006. Oligosaccharide structures studied by hydrogen–deuterium exchange and MALDI-TOF mass spectrometry. Anal. Chem. 78 (15), 5302–5308.
Green-Church K.B., Limbach P.A., Freitas M.A., Marshall A.G. 2001. Gas-phase hydrogen/deuterium exchange of positively charged mononucleotides by use of Fourier-transform ion cyclotron resonance mass spectrometry. J. Am. Soc. Mass Spectrom. 12 (3), 268–277.
Balbeur D., Widart J., Leyh B., Cravello L., de Pauw E. 2008. Detection of oligonucleotide gas-phase conformers: H/D exchange and ion mobility as complementary techniques. J. Am. Soc. Mass Spectrom. 19 (7), 938–946.
Solouki T., Freitas M.A., Alomary A. 1999. Gas-phase hydrogen/deuterium exchange reactions of fulvic acids: An electrospray ionization Fourier transform ion cyclotron resonance mass spectral study. Anal. Chem. 71 (20), 4719–4726.
Kostyukevich Y., Kononikhin A., Popov I., Nikolaev E. 2013. Simple atmospheric hydrogen/deuterium exchange method for enumeration of labile hydrogens by electrospray ionization mass spectrometry. Anal. Chem. 85 (11), 5330–5334.
Hemling M.E., Conboy J. J., Bean M. F., Mentzer M., Carr S.A. 1994. Gas-phase hydrogen-deuterium exchange in electrospray-ionization mass-spectrometry as a practical tool for structure elucidation. J. Am. Soc. Mass Spectrom. 5 (5), 434–442.
Takats Z., Nanita S.C., Schlosser G., Vekey K., Cooks R.G. 2003. Atmospheric pressure gas-phase H/D exchange of serine octamers. Anal. Chem. 75 (22), 6147–6154.
Kostyukevich Y., Kononikhin A., Popov I., Spasskiy A., Nikolaev E. 2015. In ESI-source H/D exchange under atmospheric pressure for peptides and proteins of different molecular weights from 1 to 66 kDa: The role of the temperature of the desolvating capillary on H/D exchange. J. Mass Spectrom. 50 (1), 49–55.
Kostyukevich Y., Kononikhin A., Popov I., Nikolaev E. 2014. In-ESI source hydrogen/deuterium exchange of carbohydrate ions. Anal. Chem. 86 (5), 2595–2600.
Kostyukevich Y., Kononikhin A., Popov I., Starodubtseva N., Kukaev E., Nikolaev E. 2014. Letter: Separation of tautomeric forms of [2-nitrophloroglucinol-H](–) by an in-electrospray ionization source hydrogen/deuterium exchange approach. Eur. J. Mass Spectrom. 20 (4), 345–349.
Kostyukevich Y., Kononikhin A., Popov I., Starodubtzeva N., Pekov S., Kukaev E., Indeykina M., Nikolaev E. 2015. Analytical potential of the in-electrospray ionization source hydrogen/deuterium exchange for the investigation of oligonucleotides. Eur. J. Mass Spectrom. 21 (1), 59–63.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Yu.I. Kostyukevich, A.S. Kononikhin, M.I. Indeykina, I.A. Popov, K.V. Bocharov, A.I. Spassky, S.A. Kozin, A.A. Makarov, E.N. Nikolaev, 2017, published in Molekulyarnaya Biologiya, 2017, Vol. 51, No. 4, pp. 710–716.
Rights and permissions
About this article
Cite this article
Kostyukevich, Y.I., Kononikhin, A.S., Indeykina, M.I. et al. Secondary structure of Aβ(1–16) complexes with zinc: A study in the gas phase using deuterium/hydrogen exchange and ultra-high-resolution mass spectrometry. Mol Biol 51, 627–632 (2017). https://doi.org/10.1134/S0026893317030104
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893317030104