Abstract
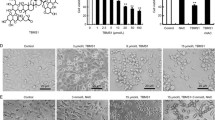
Mitochondria play central roles in diverse physiological and pathological conditions associated with cell survival and death. Delocalized lipophilic cations, such as dequalinium (DQA), are accumulated in cancer cells attracted by the highly negative mitochondrial transmembrane potential of these cells. DQA showed a potent anticancer activity in cells from different malignancies. Here, we report the effect of DQA on PC-3 prostate cancer cells. Incubation with DQA at concentrations between 1.5 and 100 μM from 24 to 48 h decreases cell viability. The decrease in cell viability together with a loss of mitochondrial transmembrane potential induced an increase in reactive oxygen species production and cell death via caspase-3 dependent apoptotic pathway. DQA was shown to cause moderate to strong cell death in a time and concentration dependent manner, causing a most advantageous effect at a concentration of 10 μM applied for a long 48 h time period, which might be a consequence of the kinetics of intracellular DQA accumulation in mitochondria, but also of the mechanisms of DQA-induced cell death. This data shows DQA as a promising agent against the human prostate cancer PC-3 cell line, activating the caspase-3 dependent apoptotic pathway. This fact might be beneficial for possible future applications in cancer therapy.
Similar content being viewed by others
References
Stavridi F., Karapanagiotou E.M., Syrigos K.N. 2010. Targeted therapeutic approaches for hormone-refractory prostate cancer. Cancer Treat. Rev. 36, 122–130.
Kaighn M.E., Narayan K.S., Ohnuki Y., Lechner J.F., Jones L.W. 1979. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest. Urol. 17, 16–23.
Duchen M.R. 2004. Mitochondria in health and disease: Perspectives on a new mitochondrial biology. Mol. Aspects Med. 25, 365–451.
Bras M., Queenan B., Susin S.A. 2005. Programmed cell death via mitochondria: Different modes of dying. Biochemistry. 70, 231–239.
Vanden Berghe T., Grootjans S., Goossens V., Dondelinger Y., Krysko D.V., Takahashi N., Vandenabeele P. 2013. Determination of apoptotic and necrotic cell death in vitro and in vivo. Methods. 61, 117–129.
FerrÍn G., Linares C.I., Montané J. 2011. Mitochondrial drug targets in cell death and cancer. Curr. Pharm. Design. 17, 2002–2016.
Wen S., Zhu D., Huang P. 2013. Targeting cancer cell mitochondria as a therapeutic approach. Future Med. Chem. 5, 53–67.
Biasutto L., Dong L.-F., Zoratti M., Neuzil J. 2010. Mitochondrially targeted anti-cancer agents. Mitochondrion. 10, 670–681.
Wang F., Ogasawara M.A., Huang P. 2010. Small mitochondria-targeting molecules as anti-cancer agents. Mol. Aspects. Med. 31, 75–92.
Zhang E., Zhang C., Su Y., Cheng T., Shi C. 2011. Newly developed strategies for multifunctional mitochondria-targeted agents in cancer therapy. Drug. Discov. Today. 16, 140–146.
Modica-Napolitano J.S., Aprille J.R. 2001. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv. Drug. Deliv. Rev. 49, 63–70.
Christman J.E., Miller D.S., Coward P., Smith L.H., Teng N. 1990. Study of the selective cytotoxic properties of cationic, lipophilic mitochondrial-specific compounds in gynecologic malignancies. Gynecol. Oncol. 39, 72–79.
Helige C., Smolle J., Zellnig G., Fink-Puches R., Kerl H., Tritthart H.A. 1992. Effect of dequalinium on K1735-M2 melanoma cell growth, directional migration and invasion in vitro. Eur. J. Cancer. 29A, 124–128.
Abdul M., Hoosein N. 2002. Expression and activity of potassium ion channels in human prostate cancer. Cancer Lett. 186, 99–105.
Modica-Napolitano J.S., Nalbandian R., Kidd M.E., Nalbandian A., Nguyen C.C. 2003. The selective in vitro cytotoxicity of carcinoma cells by AZT is enhanced by concurrent treatment with delocalized lipophilic cations. Cancer Lett. 198, 59–68.
Sancho P., Galeano E., Nieto E., Delgado M.D., Garcia-Perez A.I. 2007. Dequalinium induces cell death in human leukemia cells by early mitochondrial alterations which enhance ROS production. Leuk. Res. 31, 969–978.
Pajuelo L., Calviño E., Diez J.C., Boyano-Adánez M.C., Gil J., Sancho P. 2010. Dequalinium induces apoptosis in peripheral blood mononuclear cells isolated from human chronic lymphocytic leukemia. Invest. New Drugs. 29, 1156–1163.
Garcia-Perez A.I., Galeano E., Nieto E., Sancho P. 2011. Dequalinium induces human leukemia cell death by affecting the redox balance. Leuk. Res. 35, 1395–1401.
Weissig V., Torchilin V.P. 2001. Towards mitochondria gene therapy: DQAsomes as a strategy. J. Drug Target. 9, 1–13.
Lyrawati D., Trounson A., Cram D. 2011. Expression of GFP in the mitochondrial compartment using DQAsome-mediated delivery of an artificial minimitochondrial genome. Pharm. Res. 28, 2848–862.
Weissig V., Lizano C., Torchilin V. 1998. Micellar delivery system for dequalinium A lipophilic cationic drug with anticarcinoma activity. J. Liposome Res. 8, 391–400.
Green M.R., Sambrook J. 2012. Molecular Cloning: A Laboratory Manual, 4th ed. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press.
Gonzalez-Guerrico A.M., Meshki J., Xiao L., Benavides F., Conti C.J., Kazanietz M.G. 2005. Molecular mechanisms of protein kinase C-induced apoptosis in prostate cancer cells. J. Biochem. Mol. Biol. 38, 639–645.
Decker P., Muller S. 2002. Modulating poly (ADPribose) polymerase activity: Potential for the prevention and therapy of pathogenic situations involving DNA damage and oxidative stress. Curr. Pharm. Biotechnol. 3, 275–283.
Huang Y.T., Chueh S.C., Teng C.M., Guh J.H. 2004. Investigation of ouabain-induced anticancer effect in human androgen-independent prostate cancer PC-3 cells. Biochem. Pharmacol. 67, 727–733.
Liu H., Liu Y.Q., Liu Y.Q., Xu A.H., Young C.Y., Yuan H.Q., Lou H.X. 2010. A novel anticancer agent, retigeric acid B, displays proliferation inhibition, S phase arrest and apoptosis activation in human prostate cancer cells. Chem. Biol. Interact. 188, 598–606.
Xu A.H., Hu Z.M., Qu J.B., Liu S.M., Syed A.K., Yuan H.Q., Lou H.X. 2010. Cyclic bisbibenzyls induce growth arrest and apoptosis of human prostate cancer PC-3 cells. Acta Pharmacol. Sin. 31, 609–615.
Zhang X.Q., Huang X.F., Mu S.J., An Q.X., Xia A.J., Chen R., Wu D.C. 2010. Inhibition of proliferation of prostate cancer cell line, PC-3, in vitro and in vivo using (−)-gossypol. Asian J. Androl. 12, 390–399.
Patra N., De U., Kang J.A., Kim J.M., Ahn M.Y., Lee J., Jung J.H., Chung H.Y., Moon H.R., Kim H.S. 2011. A novel epoxypropoxy flavonoid derivative and topoisomerase II inhibitor, MHY336, induces apoptosis in prostate cancer cells. Eur. J. Pharmacol. 658, 98–107.
Samarghandian S., Afshari J.T., Davoodi S. 2011. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line PC-3. Clinics (São Paulo). 66, 1073–1079.
Senthilkumar K., Arunkumar R., Elumalai P., Sharmila G., Gunadharini D.N., Banudevi S., Krishnamoorthy G., Benson C.S., Arunakaran J. 2011. Quercetin inhibits invasion, migration and signalling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3). Cell Biochem. Funct. 29, 87–95.
Martin S.J., Reutelingsperger C.P., McGahon A.J., Rader J.A., van Schie R.C., LaFace D.M., Green D.R. 1995. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182, 1545–1556.
Pradelli L.A., Bénéteau M., Ricci J.E. 2010. Mitochondrial control of caspase-dependent and -independent cell death. Cell. Mol. Life Sci. 67, 1589–1597.
Agarwal A., Mahfouz R.Z., Sharma R.K., Sarkar O., Mangrola D., Mathur P.P. 2009. Potential biological role of poly (ADP-ribose) polymerase (PARP) in male gametes. Reprod. Biol. Endocrinol. 7, 143–163.
Bataller M., Portugal J. 2005. Apoptosis and cell recovery in response to oxidative stress in p53-deficient prostate carcinoma cells. Arch. Biochem. Biophys. 437, 151–158.
Valko M., Rhodes C.J., Moncol J., Izakovic M., Mazur M. 2006. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160, 1–40.
Paschos A., Pandya R., Duivenvoorden W.C., Pinthus J.H. 2013. Oxidative stress in prostate cancer: Changing research concepts towards a novel paradigm for prevention and therapeutics. Prostate Cancer Prostatic Dis. 16, 217–225.
Pelicano H., Carney D., Huang P. 2004. ROS stress in cancer cells and therapeutic implications. Drug Resist. Update. 7, 97–110.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Molekulyarnaya Biologiya, 2014, Vol. 48, No. 3, pp. 416–428.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Makowska, K., Estañ, M.C., Gañán-Gómez, I. et al. Changes in mitochondrial function induced by dequalinium precede oxidative stress and apoptosis in the human prostate-cancer cell line PC-3. Mol Biol 48, 359–370 (2014). https://doi.org/10.1134/S0026893314030133
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893314030133