Abstract
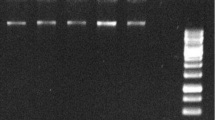
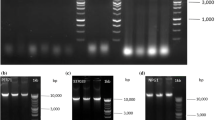
Abelmoschus esculentus (okra) is one of the polysaccharide rich crop plants. The polysaccharides interfere with nucleic acids and protein isolation thereby affecting the downstream molecular analysis. So, to understand the molecular systematics of okra, high quality DNA, RNA and proteins are essential. In this study we present a method for extracting genomic DNA, RNA and proteins from polysaccharide rich okra tissues. The conventional extraction procedures were integrated with purification treatments with pectinase, RNase and proteinase K, which improved the quality and quantity of DNA as well. Using SDS, additional washes with CIA and NaCl precipitation improved the RNA isolation both quantitatively and qualitatively. Finally, ammonium acetate mediated protein precipitation and re-solubilization increased the quality of total protein extracts from the okra leaves. All of the methods above not only eliminated the impurities but also improved the quality and quantity of nucleic acids and proteins. Further, we subjected these samples to versatile downstream molecular analyses such as restriction endonuclease digestion, RAPD, Southern, reverse transcription-PCR and Western analysis and were proved to be successful.
Similar content being viewed by others
Abbreviations
- PC:
-
phenol (pH 4.5): chloroform
- CIA:
-
chloroform: isoamyl alcohol
- PCIA:
-
phenol: chloroform: isoamyl alcohol
- CTAB:
-
hexadecyltrimethylammonium bromide
- DTT:
-
dithiotreitol
- PMSF:
-
phenylmethanesulfonyl fluoride
- PVPP:
-
polyvinyl polypyrrolidone
- DEPC:
-
diethyl pyrocarbonate
- RH:
-
relative humidity
- RT:
-
room temperature
References
Porebski S., Bailey L.G., Baum B.R. 1997. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharides and polyphenol component. Plant Mol. Biol. Rep. 15, 8–15.
Fang G., Hammar S., Grumet R. 1992. A quick and inexpensive method for removing polysaccharides from plant genomic DNA. BioTechniques. 13, 52–54.
Csaikl U.M., Bastian H., Brettschneider S., Gauch A., Meir M., Schauerte F., Scholz S., Sperisen B., Vornam Z.B. 1998. Comparative analysis of different DNA extraction protocols: A fast, universal maxi-preparation of high quality plant DNA for genetic evaluation and phylogenetic studies. Plant Mol. Biol. Rep. 16, 69–86.
Belletti P.C., Marzachi K., Lanteri S. 1998. Flow cytometric measurement of nuclear DNA content in Capsicum (Solanaceae). Plant Syst. Evol. 209, 85–91.
Schlink K., Reski R. 2002. Preparing high quality DNA from moss (Physcomitrella parens). Plant. Mol. Biol. Rep. 20, 423–423.
Yun-Jiang C., Wen-Wu G., Hva-Lin Y., Xlao-Min P., Xivxin D. 2003. An efficient protocol for genomic DNA extraction from citrus species. Plant Mol. Biol. Rep. 21, 177–177.
Michaud H., Lumaret J.P., Ripoll L.T. 1995. A procedure for the extraction of chloroplast DNA from broad-leaved tree species. Plant Mol. Biol. Rep. 2, 131–137.
Tribouch S.O., Danilenko N.G., Davydenko O.G. 1998. A method for isolation of chloroplast DNA and mitochondrial DNA from sunflower. Plant Mol. Biol. Rep. 16, 183–189.
Scott K.D., Playford J. 1996. DNA Extraction technique for PCR in rainforest species. BioTechniques. 20, 974–978.
Shepherd M., Cross M., Stokoe R.L., Scott L.J., Jones E.M. 2002. High throughput DNA extraction from forest trees. Plant Mol. Biol. Rep. 20, 425–425.
Bhattacharjee R., Maria K.A., Peter A., Sunday T., Ivan I. 2004. An improved semi automated rapid method of extracting genomic DNA for molecular marker analysis in cocoa, Theobroma cacao L. Plant Mol. Biol. Rep. 22, 435a–435b.
Doyle J.J., Doyle J.L. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15.
Doyle J.J., Doyle J.L. 1990. A rapid total DNA preparation procedure for fresh plant tissue. Focus. 12, 13–15.
Huang J., Ge X., Sun M. 2000. Modified CTAB protocol using a silica matrix for isolation of plant genomic DNA. BioTechniques. 28, 432–434.
Marechal-Drouard L., Guillemaut P. 1995. A powerful but simple technique to prepare polysaccharide-free DNA quickly and without phenol extraction. Plant Mol. Biol. Rep. 13, 26–30.
Suzuki Y., Makino A.A., Mae T. 2001. An efficient method for extraction of RNA from rice leaves at different ages using benzyl chloride. J. Exp. Bot. 52(360), 1575–1579.
Tanaka J., Ikeda S. 2002. Rapid and efficient DNA extraction method from various plant species using diatomaceous earth and a spin filter. Breeding Sci. 52, 151–155.
Jobes D.V., Hurley D.L., Thien L.B. 1995. Plant DNA isolation: A method to efficiently remove polyphenolics, polysaccharides, and RNA. Taxon. 44, 379–386.
Wang W., Tai F., Chen S. 2008. Optimizing protein extraction from plant tissues for enhanced proteomics analysis. J. Separ. Sci. 31(11), 2032–2039.
Lee J., Cooper B. 2006. Alternative workflows for plant proteomic analysis. Mol. Biosyst. 2(12), 621–626.
Görg A., Obermaier C., Boguth G., Harder A., Scheibe B., Wildgruber R., Weiss W. 2000. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 21, 1037–1053.
Franklin W., Martin. 1982. Okra, potential multiple-purpose crop for the temperate zones and tropics. Econ. Bot. 36, 340–345.
Sengkhamparn N., Verhoef R., Henk A.S., Sajjaanantakul T., Voragen A.J.G. 2009. Characterisation of cell wall polysaccharides from okra, Abelmoschus esculentus (L.) Moench. Carbohydr. Res. 344(14), 1824–1832.
Sambrook J., Russell D.W. 2001. Molecular Cloning. A Laboratory Manual, 3rd ed. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press.
Church G.M., Gilbert W. 1984. Genomic sequencing. Proc. Natl. Acad. Sci. U. S. A. 81, 1991–1995.
Williams J.G.K., Kubelik A.R., Livak K.L., Rafalski J.A., Tingey S.V. 1990. DNA polymorphism amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 18, 6531–6535.
Manoj-Kumar A., Kalpana-Reddy N., Luke Simon, Ramachandra Y.L. 2009. RAPD analysis of local bell pepper genotypes in relation with powdery mildew incidence and fruit yield: Managing disease by single chemical molecule. Arch. Phytopathol. Plant Protect. 42(9), 891–901.
Hurkman W.J., Tanaka C.K. 1986. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 81, 802–806.
Bradford M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254.
Chomczynski P., Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanatephenol-chloroform extraction. Anal. Biochem. 162, 156–159.
Loomis W.D. 1974. Overcoming problems of phenolic and quinones in the isolation of plant enzymes and organelles. Methods Enzymol. 31, 528–545.
Harwood A.J. 1996. Basic DNA and RNA protocols. Methods Mol. Biol. 58, 3–9.
Rogstad S.H., Keane B., Keiffer C.H., Hebard F., Sisco P. 2001. DNA extraction from plants: The use of pectinase. Plant Mol. Biol. Rep. 19, 353–359.
Bhatnagar S.K., Deepika A., Sanjeev Kumar. 2005. First protocol for DNA isolation in Indian charophyta. J. Biol. Res. 3, 109–111.
Caramante M., Corrado G., Maria Monti L., Rosa Rao. 2011. Simple sequence repeats are able to trace tomato cultivars in tomato food chains. Food Control. 22, 549–554.
Bardakci F. 2001. Random Amplified Polymorphic DNA (RAPD) markers. Turk. J. Biol. 25, 185–196.
Claros G.M., Francisco M., Canovas M. 1999. Experimental section: RNA isolation from plant tissues: A practical experience for biological undergraduates. Biochem. Educ. 27, 110–113.
Barry R., Soloviev M. 2004. Quantitative protein profiling using antibody arrays. Proteomics. 4, 3717–3726.
Espagne C., Martinez A., Valot B., Meinnel T., Giglione C. 2007. Alternative and effective proteomic analysis in Arabidopsis. Proteomics. 7, 3788–3799.
Kalpana-Reddy N., Kumar A., Rajanna M.D., Rahiman A., Gowda B. 2009. Evaluation of maize genotypes against downy mildew (Peronosclerospora sorghi (Weston and Uppal)) and characterization of soluble seed proteins by SDS-PAGE. Arch. Phytopathol. Plant Protect. 42(12), 1126–1131.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Manoj-Kumar, A., Reddy, K.N., Manjulatha, M. et al. Polysaccharide-free nucleic acids and proteins of Abelmoschus esculentus for versatile molecular studies. Mol Biol 46, 535–541 (2012). https://doi.org/10.1134/S0026893312030065
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893312030065