Abstract
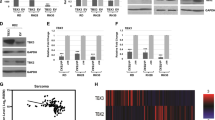
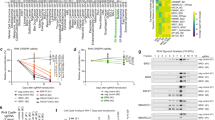
Coordination of proliferation and differentiation in cells committed to muscle fate requires the interaction of the retinoblastoma gene product (pRb) with transcription factors of the E2F family, pRb having different affinities for distinct E2Fs. It was found that pRb carrying a small deletion at the end of the T antigen-binding region (ΔS/N) and unable to interact with the SV40 large T antigen could induce an acute cell cycle block, stable prolongation of the cell cycle in G0/G1 and G2/M phases, and suppression of the growth of tumor cells. ΔS/N showed increased affinity for E2F4, bound hyperphosphorylated forms of E2F4, and induced its nuclear compartmentalization. The ability of ΔS/N to form complexes with E2F4 on DNA was associated with an increased formation of “free” E2F4 and trans-suppression of a specific reporter through preferential binding to E2F4, but not to E2F1. Stable expression of ΔS/N in multipotent fibroblasts promoted early muscle commitment. It was assumed that a mutation in the T antigen-binding region increases pRb affinity for E2F4 combined with activation of muscle differentiation.
Similar content being viewed by others
Abbreviations
- PCR:
-
polymerase chain reaction
- pRb:
-
product of gene of retinoblastome
- BrdU:
-
bromdeoxyuridine
- CdK:
-
cyclin-dependent kinase
- EMSA:
-
method of shift of electrophoretic mobility in gel
- FCS:
-
fetal serum of cattle
- PBS:
-
phosphate buffer
- BSA:
-
bovine serum albumin
- pp-E2F:
-
comlex “pocket” proteins-E2F-DNA
- pp-E2F:
-
comlex “pocket” proteins E2F-cyclin E-A/Cdk2-DNA
- SDS-PAGE:
-
electrophoresis with sodium dodecylsulfate in polyacrylamide gel
References
Skapek S.X., Pan Y.R., Lee E.Y. 2006. Regulation of cell lineage specification by the retinoblastoma tumor suppressor. Oncogene. 25, 268–5276.
Otterson G.A., Chen W., Coxon A.B., et al. 1997. Incomplete penetrance of familial retinoblastoma linked to germ-line mutations that result in partial loss of RB function. Proc. Natl. Acad. Sci. USA. 94, 12036–12040.
Sellers W.R., Novitch B.G., Miyake S., et al. 1998. Stable binding to E2F is not required for the retinoblastoma protein to activate transcription, promote differentiation, and suppress tumor cell growth. Genes Dev. 12, 95–106.
Frolov M.V., Dyson N.J. 2004. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. J. Cell Sci. 117, 2173–2181.
Brown V.D., Gallie B.L. 1999. Cumulative effect of phosphorylation of pRB on regulation of E2F activity. Mol. Cell. Biol. 19, 3246–3256.
Lee J.O., Russo A.A., Pavletich N.P. 1998. Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7. Nature. 391, 859–865.
Rubin S.M., Gall A.L., Zheng N., et al. 2005. Structure of the Rb C-terminal domain bound to E2F1-DP1: A mechanism for phosphorylation-induced E2F release. Cell. 123, 1093–1106.
Harbour J.W., Luo R.X., Dei Santi A., et al. 1999. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 98, 859–869.
Brown V.D., Gallie B.L. 2002. The B-domain lysine patch of pRB is required for binding to large T antigen and release of E2F by phosphorylation. Mol. Cell Biol. 22, 1390–1401.
Blais A., Dynlacht B.D. 2007. E2F-associated chromatin modifiers and cell cycle control. Curr. Opin. Cell. Biol. 19, 658–662.
Shan B., Durfee T., Lee W.H. 1996. Disruption of RB/E2F-1 interaction by single point mutations in E2F-1 enhances S-phase entry and apoptosis. Proc. Natl. Acad. Sci. USA. 93, 679–684.
Moberg K., Starz M.A., Lees J.A. 1996. E2F-4 switches from p130 to p107 and pRb in response to cell cycle reentry. Mol. Cell Biol. 16, 1436–1449.
Popov B., Chang L-S., Serikov V. 2005. Cell cycle-related transformation of the E2F4-p130 repressor complex. Biochem. Biophys. Res. Commun. 336, 762–769.
Black B.L., Molkentin J.D., Olson E.N. 1998. Multiple roles for the MyoD basic region in transmission of transcriptional activation signals and interaction with MEF2. Mol. Cell Biol. 18, 69–77.
Novitch B.G., Mulligan G.J., Jacks T., et al. 1996. Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J. Cell Biol. 135, 441–456.
Huh M.S., Parker M.H., Scime A., et al. 2004. Rb is required for progression through myogenic differentiation but not maintenance of terminal differentiation. J. Cell Biol. 166, 865–876.
Hamel P.A., Cohen B.L., Sorce L.M., et al. 1990. Hyperphosphorylation of the retinoblastoma gene product is determined by domains outside the simian virus 40 large-T-antigen-binding regions. Mol. Cell Biol. 10, 6586–6595.
Hamel P.A., Gill R.M., Phillips R.A. et al. 1992. Regions controlling hyperphosphorylation and conformation of the retinoblastoma gene product are independent of domains required for transcriptional repression. Oncogene. 7, 693–701.
Constantinides P.G., Jones P.A., Gevers W. 1977. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 267, 364–366.
Muller H., Moroni M.C., Vigo E., et al. 1997. Induction of S-phase entry by E2F transcription factors depends on their nuclear localization. Mol. Cell Biol. 17, 5508–5520.
Vairo G., Livingston D.M., Ginsberg D. 1995. Functional interaction between E2F-4 and p130: Evidence for distinct mechanisms underlying growth suppression by different retinoblastoma protein family members. Genes Dev. 9, 869–881.
Dick F.A. 2007. Structure-function analysis of the retinoblastoma tumor suppressor protein: Is the whole a sum of its parts? Cell Div. 2, 26–41.
Lee C., Chang J.H., Lee H.S., et al. 2002. Structural basis for the recognition of the E2F transactivation domain by the retinoblastoma tumor suppressor. Genes Dev. 16, 3199–3212.
Sun H., Chang Y., Schweers B., et al. 2006. An E2F binding-deficient Rb1 protein partially rescues developmental defects associated with Rb1 nullizygosity. Mol. Cell Biol. 26, 1527–1537.
Popov B.V., Kulakova I.A., Popov N.B., et al. 1998. Phosphorylation pattern of the retinoblastoma gene product in stable murine and human cell lines. Ontogenez. 29, 245–253.
Ren B., Cam H., Takahashi Y., et al. 2002. E2F integrates cell cycle progression with DNA repair, replication, and G2/M checkpoints. Genes Dev. 16, 245–256.
Hateboer G., Kerkhoven R.M., Shvarts A., et al. 1996. Degradation of E2F by the ubiquitin-proteasome pathway: Regulation by retinoblastoma family proteins and adenovirus transforming proteins. Genes Dev. 10, 2960–2970.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © B.V. Popov, S.M. Watt, J.M. Rosanov, L.-S. Chang, 2010, published in Molekulyarnaya Biologiya, 2010, Vol. 44, No. 2, pp. 323–334.
Rights and permissions
About this article
Cite this article
Popov, B.V., Watt, S.M., Rosanov, J.M. et al. A structural pocket mutation of pRb increases its affinity for E2F4, which is coupled with activation of muscle differentiation. Mol Biol 44, 287–297 (2010). https://doi.org/10.1134/S0026893310020147
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893310020147