Abstract
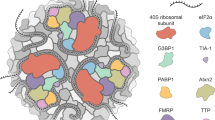
The review considers recent data on stress granules, which are dense RNP-containing cytoplasmic bodies that arise under stress conditions, e.g., in heat shock, UV irradiation, energy depletion, and oxidative stress. There is evidence that stress granules accumulate incomplete initiation complexes containing mRNA associated with proteins, small ribosomal subunits, and some translation initiation factors, and that stress granules are formed when cells are depleted of the ternary complex (eIF2-tRNAMet-GTP), in particular, upon eIF2A phosphorylation or a decrease in GTP. Large ribosomal subunits and the ternary complex are absent from stress granules. The structural basis of stress granules is known. It is probable, however, that RNA-binding protein TIA-1, which normally occurs in the nucleus, forms prion-like aggregates that serve as scaffolds for other components of stress granules. The cytoskeleton facilitates the accumulation of stress granule components in local cytoplasmic sites. Studies of the formation and composition of stress granules are important for a better understanding of the regulation of translation initiation in vivo and the mechanisms of the cell response to stress factors.
Similar content being viewed by others
References
Nover L., Scharf K.D., Neumann D. 1983. Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol. Cell. Biol. 3, 1648–1655.
Kedersha N., Chen S., Gilks N., Li W., Miller I.J., Stahl J., Anderson P. 2002. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol. Biol. Cell. 13, 195–210.
Kimball S.R., Horetsky R.L., Ron D., Jefferson L.S., Harding H.P. 2003. Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am. J. Physiol. Cell Physiol. 10, 524–530.
Kedersha N.L., Gupta M., Li W., Miller I., Anderson P. 1999. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2α to the assembly of mammalian stress granules. J. Cell Biol. 147, 1431–1442.
Kim W.J., Back S.H., Kim V., Ryu I., Jang S.K. 2005. Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions. Mol. Cell Biol. 25, 2450–2462.
Kedersha N., Anderson P. 2002. Stress granules: Sites of mRNA triage that regulate mRNA stability and translatability. Biochem. Soc. Trans. 30, 963–969.
Kedersha N., Cho M.R., Li W., Yacono P.W., Chen S., Gilks N., Golan D.E., Anderson P. 2002. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 151, 1257–1268.
Kedersha N., Stoecklin G., Ayodele M., Yacono P., Lykke-Andersen J., Fitzler M.J., Scheuner D., Kaufman R.J., Golan D.E., Anderson P. 2005. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 169, 871–884.
Mazroui R., Huot M.E., Tremblay S., Filion C., Labelle Y., Khandjian E.W. 2002. Trapping of messenger RNA by Fragile X Mental Retardation protein into cytoplasmic granules induces translation repression. Hum. Mol. Genet. 11, 3007–3017.
Thomas M.G., Martinez Tosar L.J., Loschi M., Pasquini J.M., Correale J., Kindler S., Boccaccio G.L. 2005. Staufen recruitment into stress granules does not affect early mRNA transport in oligodendrocytes. Mol. Biol. Cell. 16, 40–420.
Murata T., Morita N., Hikita K., Kiuchi K., Kiuchi K., Kaneda N. 2005. Recruitment of mRNA-destabilizing protein TIS11 to stress granules is mediated by its zinc finger domain. Exp. Cell Res. 303, 287–299.
Tourriere H., Chebli K., Zekri L., Courselaud B., Blanchard J.M., Bertrand E., Tazi J. 2003. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 160, 823–831.
Le Guiner C., Lejeune F., Galiana D., Kister L., Breathnach R., Stevenin J., Del Gatto-Konczak F. 2001. TIA-1 and TIAR activate splicing of alternative exons with weak 5′ splice sites followed by a U-rich stretch on their own pre-mRNAs. J. Biol. Chem. 276, 40,638–40,646.
Piecyk M., Wax S., Beck A.R., Kedersha N., Gupta M., Maritim B., Chen S., Gueydan C., Kruys V., Streuli M., Anderson P. 2000. TIA-1 is a translational silencer that selectively regulates the expression of TNFα. EMBO J. 19, 4154–4163.
Dixon D.A., Balch G.C., Kedersha N., Anderson P., Zimmerman G.A., Beauchamp R.D., Prescott S.M. 2003. Regulation of cyclooxygenase-2 expression by the translational silencer TIA-1. J. Exp. Med. 198, 475–481.
Lopez de Silanes I., Galban S., Martindale J.L., Yang, X., Mazan-Mamczarz K., Indig F.E., Falco G., Zhan M., Gorospe M. 2005. Identification and functional outcome of mRNAs associated with RNA-binding protein TIA-1. Mol. Cell. Biol. 25, 9520–9531.
Gilks N., Kedersha N., Ayodele M., Shen L., Stoecklin G., Dember L.M., Anderson P. 2004. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell. 15, 5383–5398.
Anderson P., Kedersha N. 2002. Stressful initiations. J. Cell Sci. 15, 3227–3234.
Ter-Avanesyan M.D., Dagkesamanskaya A.R., Kushnirov V.V., Smirnov V.N. 1994. The SUP35 omnipotent suppressor gene is involved in the maintenance of the non-Mendelian determinant [psi +] in the yeast Saccharomyces cerevisiae. Genetics. 137, 671–676.
Zhouravleva G., Frolova L., Le Goff X., Le Guellec R., Inge-Vechtomov S., Kisselev L., Philippe M. 1995. Termination of translation in eukaryotes is governed by two interacting polypeptide chain release factors, eRF1 and eRF3. EMBO J. 14, 4065–4072.
Crosby J.S., Chefalo P.J., Yeh I., Ying S., London I.M., Leboulch P., Chen J.J. 2000. Regulation of hemoglobin synthesis and proliferation of differentiating erythroid cells by heme-regulated eIF-2α kinase. Blood. 96, 3241–3248.
Lu L., Han A.P., Chen J.J. 2001. Translation initiation control by heme-regulated eukaryotic initiation factor 2α kinase in erythroid cells under cytoplasmic stresses. Mol. Cell. Biol. 23, 7971–7980.
Mathews M.B. 1993. Viral evasion of cellular defense mechanisms: Regulation of the protein kinase DAI by RNA effectors. Semin. Virol. 4, 247–257.
Harding H.P., Zhang Y., Ron D. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 397, 271–274.
Wu S., Hu Y., Wang J.L., Chatterjee M., Shi Y., Kaufman R.J. 2002. Ultraviolet light inhibits translation through activation of the unfolded protein response kinase PERK in the lumen of the endoplasmic reticulum. J. Biol. Chem. 277, 18,077–18,083.
Jiang H.Y., Wek R.C. 2005. GCN2 phosphorylation of eIF2α activates NF-κB in response to UV irradiation. Biochem. J. 385, 371–380.
Patel J., McLeod L.E., Vries R.G., Flynn A., Wang X., Proud C.G. 2002. Cellular stresses profoundly inhibit protein synthesis and modulate the states of phosphorylation of multiple translation factors. Eur. J. Biochem. 269, 3076–3085.
Luby-Phelps K., Castle P.E., Taylor D.L., Lanni F. 1987. Hindered diffusion of inert tracer particles in the cytoplasm of mouse 3T3 cells. Proc. Natl. Acad. Sci. USA. 84, 4910–4913.
Ivanov P.A., Chudinova E.M., Nadezhdina E.S. 2003. Disruption of microtubules inhibits cytoplasmic ribonucleoprotein stress granule formation. Exp. Cell Res. 290, 227–233.
Knowles R.B., Sabry J.H., Martone M.E., Deerinck T.J., Ellisman M.H., Bassell G.J., Kosik K.S. 1996. Translocation of RNA granules in living neurons. J. Neurosci. 16, 7812–7820.
Carson J.H., Worboys K., Ainger K., Barbarese E. 1997. Translocation of myelin basic protein mRNA in oligodendrocytes requires microtubules and kinesin. Cell Motil. Cytoskel. 38, 318–328.
Fusco D., Accornero N., Lavoie B., Shenoy S.M., Blanchard J.M., Singer R.H., Bertrand E. 2003. Single mRNA molecules demonstrate probabilistic movement in living mammalian cells. Curr. Biol. 13, 161–167.
Gowrishankar G., Winzen R., Bollig F., Ghebremedhin B., Redich N., Ritter B., Resch K., Kracht M., Holtmann H. 2005. Inhibition of mRNA deadenylation and degradation by ultraviolet light. Biol. Chem. 386, 1287–1293.
Sheth U., Parker R. 2003. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science. 300, 753–755.
Cougot N., Babajko S., Seraphin B. 2004. Cytoplasmic foci are sites of mRNA decay in human cells. J. Cell Biol. 165, 31–40.
Wilczynska A., Aigueperse C., Kress M., Dautry F., Weil D. 2005. The translational regulator CPEB1 provides a link between dcp1 bodies and stress granules. J. Cell Sci. 118, 981–992.
Author information
Authors and Affiliations
Additional information
Original Russian Text © P.A. Ivanov, E.S. Nadezhdina, 2006, published in Molekulyarnaya Biologiya, 2006, Vol. 40, No. 6, pp. 937–944.
Rights and permissions
About this article
Cite this article
Ivanov, P.A., Nadezhdina, E.S. Stress granules: RNP-containing cytoplasmic bodies arising in stress: Structure and mechanism of organization. Mol Biol 40, 844–850 (2006). https://doi.org/10.1134/S0026893306060021
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0026893306060021