Abstract
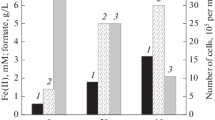
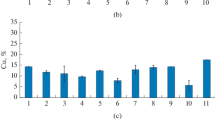
Thirty enrichment cultures of thermophilic microorganisms were obtained from Kamchatka terrestrial hydrotherms that reduced insoluble poorly crystalline Fe(III) oxide (ferrihydrite) with and without direct contact between the cells and the mineral. Restricted access to the Fe(III) mineral was achieved by incorporation of ferrihydrite into alginate beads. According to phylogenetic analysis of 22 enrichment cultures by denaturing gradient gel electrophoresis of 16S rRNA gene fragments, Firmicutes were predominant among bacteria in all the variants. Microorganisms of the phylogenetic types Aquificae, Bacteroidetes, Nitrospirae, Planctomycetes, Spirochaetes, Synergistetes, and Thermotogae were also revealed. The archaea revealed belonged to the genera Desulfurococcus, Pyrobaculum, and Thermofilum. In the case of free access to ferrihydrite, most of the phylotypes belonged to genera known for Fe(III) reduction. In the absence of direct contact with the mineral, together with known iron reducers, organisms for which ability to reduce Fe(III) was unknown were detected. Members of the genera Carboxydothermus, Thermoanaerobacter, and Thermotoga were detected most often both in the presence and absence of contact with ferrihydrite. These organisms probably possess efficient mechanisms for Fe(III) reduction within the experimental temperature range. Microbial phylogenetic diversity was higher when acetate, rather than lactate, was used as a potential electron donor.
Similar content being viewed by others
References
Lovley, D.R., and Holmes, D.E., and Nevin, K.P., Dissimilatory Fe(III) and Mn(IV) Reduction, Adv. Microb. Physiol., 2004, vol. 49, pp. 219–286.
Slobodkin, A.I., Thermophilic Microbial Metal Reduction, Mikrobiologiya, 2005, vol. 74, no. 5, pp. 581–595 [Microbiology (Engl. Transl.), vol. 74, no. 5, pp. 501–514].
Stams, A.J.M., de Bok, F., AM, Plugge C.M., van Eekert, M.H.A., Dolfing, J., and Schraa, G., Exocellular Electron Transfer in Anaerobic Microbial Communities, Environ. Microbiol., 2006, vol. 8, pp. 371–382.
Feinberg, L.F., Srikanth, R., Vachet, R.W., and Holden, J.F., Constrains of Anaerobic Respiration in the Hyperthermophilic Archaea Pyrobaculum islandicum and Pyrobaculum aerophilum, Appl. Environ. Microbiol., 2008, vol. 74, pp. 396–402.
Slobodkin, A.I., Reysenbach, A.L., Strutz, N., Dreier, M., and Wiegel, J., Thermoterrabacterium ferrireducens gen. nov., sp. nov., a Thermophilic Anaerobic Dissimilatory Fe(III)-Reducing Bacterium from a Continental Hot Spring, Int. J. Syst. Bacteriol., 1997, vol. 47, pp. 541–547.
Nevin, K.P. and Lovley, D.R., Lack of Production of Electron-Shuttling Compounds or Solubilization of Fe(III) During Reduction of Insoluble Fe(III) Oxide by Geobacter metallireducens, Appl. Environ. Microbiol., 2000, vol. 66, pp. 2248–2251.
Hungate, R.T, A Roll Tube Method for Cultivation of Strict Anaerobes, in Methods in Microbiology, Norris, J.B. and Ribbons, D.W., Eds., New York: Academic, 1969, pp. 116–132.
Reznikov, A.A., Mulikovskaya, E.P. and Sokolov, I.Yu., Metody analiza prirodnykh vod (Analytical Methods for Natural Waters), Moscow: Nedra, 1970.
Marmur, J., A Procedure for the Isolation of Desoxyribonucleic Acid from Microorganisms, J. Mol. Biol., 1961, vol. 3, pp. 208–218.
Muyizer, G., Dewaal, E.C., and Uitterlinden, A.G., Profiling of Complex Microbial Populations by Denaturing Gradient Gel Electrophoresis Analysis of Polymerase Chain Reaction-Amplified Genes Coding for 16S rRNA, Appl. Environ. Microbiol., 1993, vol. 59, no. 3, pp. 695–700.
Lane, D.J, 16S/23S rRNA Sequencing, in Nucleic Acid Techniques in Bacterial Systematics, Stackebrandt, E. and Goodfellow, M., Eds., New York: Wiley, 1991, pp. 115–175.
Muyizer, G., Teske, A., Wirsen, C.O., and Jannasch, H.W., Phylogenetic Relationships of Thiomicrospira Species and Their Identification in Deep-Sea Hydrothermal Vent Samples by Denaturing Gradient Gel Electrophoresis of 16S rDNA Fragments, Arch. Microbiol., 1995, vol. 164, no. 3, pp. 165–172.
Casamayor, E.O., Massana, R., Benlloch, S., Ovreas, L., Diez, B., Goddard, V.J., Gasol, J.M., Joint, I., Rodriguez-Valera, F., and Pedrós-Alio, C., Changes in Archaeal, Bacterial and Eukaryal Assemblages along a Salinity Gradient by Comparison of Genetic Fingerprinting Methods in a Multipond Solar Saltern, Environ. Microbiol., 2002, vol. 4, pp. 338–438.
Muyizer, G., Brinkhoff, T., Nubel, U., Santegoeds, C., Schafer, H., and Wawer, C., Denaturing Gradient Gel Electrophoresis (DGGE) in Microbial Ecology, Molecular Microbial Ecology Manual, Dordrecht: Kluwer, 1997, vol. 3.4.4, pp. 1–27.
Altschul, S.F., Gish, W., Miller, W., Myers, E.W., and Lipman, D.J., Basic Local Alignment Search Tool, J. Mol. Biol., 1990, vol. 215, pp. 403–410.
Lovley, D.R., Coates, J.D., Blunt-Harris, E.L., Phillips, E.J.P., and Woodward, J.C., Humic Substances as Electron Acceptors for Microbial Respiration, Nature, 1996, vol. 382, pp. 445–448.
Wolff-Boenisch, D. and Traina, S.J., The Effect of Desferrioxamine B, Enterobactin, Oxalic Acid, and Na-Alginate on the Dissolution of Uranyl-Treated Goetite at pH 6 and 25°C, Chem. Geol., 2007, vol. 243, pp. 357–368.
Slobodkin, A.L., Tourova, T.P., Kuznetsov, B.B., Kostrikina, N.A., Chernyh, N.A., and Bonch-Osmolovskaya, E.A., Thermoanaerobacter siderophilus sp. nov., a Novel Dissimilatory Fe(III)-Reducing, Anaerobic, Thermophilic Bacterium, Int. J. Syst. Bacteriol., 1999, vol. 49, pp. 1471–1478.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Ya.N. Nepomnyashaya, G.B. Slobodkina, T.V. Kolganova, E.A. Bonch-Osmolovskaya, A.I. Netrusov, A.I. Slobodkin, 2010, published in Mikrobiologiya, 2010, Vol. 79, No. 5, pp. 672–681.
Rights and permissions
About this article
Cite this article
Nepomnyashaya, Y.N., Slobodkina, G.B., Kolganova, T.V. et al. Phylogenetic composition of enrichment cultures of thermophilic prokaryotes reducing poorly crystalline Fe(III) oxide with and without direct contact between the cells and mineral. Microbiology 79, 663–671 (2010). https://doi.org/10.1134/S0026261710050115
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261710050115