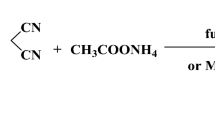Abstract
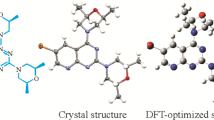
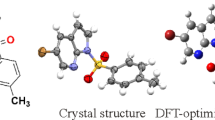
4-(2-Chlorobenzyl)-1-(5-nitro-2-(pyrrolidin-1-yl)phenyl)-[1,2,4]triazolo[4,3-a]quinazo-lin-5(4H)-one is a derivative of quinazolinones with antitumor, antibacterial, anti-inflammatory, and antimicrobial effects. Using diabetic jujube as a raw material, the title compound is synthesized by substitution and cyclization steps. The structure of the target compound is confirmed by FTIR, 1H and 13C NMR, and MS spectroscopies. The precise structure of the 4-(2-chlorobenzyl)-1-(5-nitro-2-(pyrrolidin-1-yl)phenyl)-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one compound is analyzed by single crystal X-ray diffraction (XRD). The molecular structure is further calculated using density functional theory (DFT) and the result is compared with the XRD value. The molecular electrostatic potential and frontier molecular orbitals of the title compound are investigated using DFT. In addition, the obtained atomic coordinates for the single crystal of the compound are then applied in a molecular docking simulation, and the title compound is found to participate in a number of important binding interactions in the SHP2 binding sites.







Similar content being viewed by others
REFERENCES
T. Ohtani, K. , T. , K. , Y. , T. , S. , M. , H. , and T. . Immunity, 2000, 12, 95–105. https://doi.org/10.1016/S1074-7613(00)80162-4
S. Q. , W. G. , T. , G. , L. , R. , and B. G. . Mol. Cell. Biol., 2002, 22, 4062–4072. https://doi.org/10.1128/MCB.22.12.4062-4072.2002
D. G. , D. , T. P. OSullivan, and P. J. . Tetrahedron, 2005, 61(43), 10153–10202. https://doi.org/10.1016/j.tet.2005.07.010
A. M. Bentires. Cancer Res., 2004, 64, 8816–8820. https://doi.org/10.1158/0008-5472.CAN-04-1923
M. J. Hour, L. J. Huang, S. C. Kuo, Y. K. Y. E. Hamel, and K. H. Lee. J. Med. Chem., 2000, 43, 4479–4487. https://doi.org/10.1021/jm000151c
L. , R. , M. , R. , G. , E. , L. , N. , A. , andM. . Eur. J. Pharm. Sci., 2010, 39, 428–436. https://doi.org/10.1016/j.ejps.2010.01.013
H. S. William, J. Soine, and M. Terry. Carbohydr. Res., 1989, 193, 105–113. https://doi.org/10.1016/0008-6215(89)85110-9
S. L. , Y. P. , Y. Y. Jiang, S. Y. , G. Y. Ding, and R. T. Li. Bioorg. Med. Chem. Lett., 2005, 15(7), 1915–1917. https://doi.org/10.1016/j.bmcl.2005.01.083
N. J. C. Snell. Expert Opin. Pharmacother., 2001, 2, 1317–1324. https://doi.org/10.1517/14656566.2.8.1317
A. K. C. Coello, M. G. Carril, O. M. M. López, C. M. G. , and M. A. . Rev. Cubana Farm., 2010, 44, 287–296.
X. Zhao, F. Li, W. Zhuang, X. Xue, Y. Lian, J. Fan, and D. Fang. Org. Process Res. Dev., 2010, 14, 346–350. https://doi.org/10.1021/op9002517
E. Blair, L. P. Rivory, S. J. Clarke, and A. J. Mclachlan. Br. J Clin. Pharmacol., 2015, 57, 416–426. https://doi.org/10.1111/j.1365-2125.2003.02050.x
M. D. Wodrich, C. Corminboeuf, and P. Schleyer. Org. Lett., 2006, 8, 3631–3634. https://doi.org/10.1021/ol061016i
J. H. Reibenspies and N. Bhuvanesh. Powder Diffr., 2009, 24, 347–350. https://doi.org/10.1154/1.3257614
T. Gruene, H. W. Hahn, A. V. Luebben, F. , and J. M. . J. Appl. Crystallogr., 2014, 47, 462–466. https://doi.org/10.1107/S1600576713027659
G. M. Sheldrick. Acta. Crystallogr., Sect. C, 2015, 71, 3–8. https://doi.org/10.1107/S2053273314026370
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone,B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng,J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao,H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi,M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts,R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma,V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, A. Farkas, J. B. Foresman,J. V. Ortiz, J. Cioslowski, and D. J. Fox. Gaussian09, Rev. D.01. Gaussian: Wallingford, CT, USA, 2009.
R. Dennington, T. A. Keith and J. M. Millam. GaussView, Version 5. Semichem Inc.: Shawnee Mission, KS, 2009.
S. D. Joshi, S. R. Dixit, U. A. More, K. V. S. N. , R. , T. M. , and V. H. . Med. Chem. Res., 2014, 23, 4542–4558. https://doi.org/10.1007/s00044-014-1013-1
M. I. Zavodszky, A. Rohatgi, J. R. Van Voorst, H. Yan, and L. A. Kuhn. J. Mol. Recognit., 2009, 22, 280–292. https://doi.org/10.1002/jmr.942
J. Janne, T. Huomo, T. Mikkonen, and P. . In: Lecture Notes in Business Information Processing. Springer, 2014, Vol. 182, 58–71.
C. D. Stonebanks. Stud. Symbolic Interact., 2008, 31, 207–221.
T. A. Halgren. J. Comput. Chem., 2015, 20, 730–748. https://doi.org/10.1002/(SICI)1096-987X(199905)20:7%3C730::AID-JCC8%3E3.0.CO;2-T
A. Cheng, S. A. Best, K. M. Merz, and C. H. . J. Mol. Graphics Modell., 2000, 18, 273–282. https://doi.org/10.1016/S1093-3263(00)00038-3
R. C. Newton, C. E. Manning, and J. M. Hanchar. J. Am. Ceram. Soc., 2005, 88, 1854–1858. https://doi.org/10.1111/j.1551-2916.2005.00348.x
R. Gautam and W. D. Seider. AIChE J., 1979, 25(6), 991–999. https://doi.org/10.1002/aic.690250610.
B. H. Stuart. Biological Applications. In: Infrared Spectroscopy: Fundamentals and Applications / Eds. D. J. Ando and B. H. Stuart. John Wiley & Sons, 2004, Ch. 7. https://doi.org/10.1002/0470011149.ch7
K. S. Y. Yeung, M. Hoare, N. F. Thornhill, T. , and J. D. . Biotechnol. Bioeng., 1999, 63, 684–693. https://doi.org/10.1002/(SICI)1097-0290(19990620)63:6%3C684::AID-BIT6%3E3.0.CO;2-Q
Z. Jiang, E. A. Henriksen, L. C. Tung, Y. J. Wang, M. E. Schwartz, M. Y. Han, P. Kim., and H. L. Stormer. Phys. Rev. Lett., 2007, 98(19), 197403. https://doi.org/10.1103/physrevlett.98.197403
S. W. Sharpe, T. J. Johnson, R. L. Sams, P. M. Chu, G. C. Rhoderick, and P. A. Johnson. Appl. Spectrosc., 2004, 58(12), 1452–1461. https://doi.org/10.1366/0003702042641281
Funding
The authors would like to thank the Guizhou Provincial Natural Science Foundation ([2020]1Y393).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 9, pp. 1571-1581.https://doi.org/10.26902/JSC_id80768
Rights and permissions
About this article
Cite this article
Ren, Q., Huang, P.Y., Liu, Y. et al. SYNTHESIS, CRYSTAL STRUCTURE, AND DFT STUDY OF 4-(2-CHLOROBENZYL)-1-(5-NITRO-2-(PYRROLIDIN-1-YL)PHENYL)- [1,2,4]TRIAZOLO[4,3-a]QUINAZOLIN-5(4H)-ONE. J Struct Chem 62, 1472–1482 (2021). https://doi.org/10.1134/S0022476621090171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621090171