Abstract
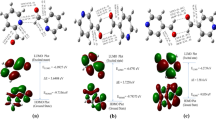
A 2-((4-(hydroxymethyl)phenyl)(pyrrolidin-1-yl)methyl)phenol molecule is a biologically important alkylaminophenol compound. In the study, this new alkylaminophenol compound is synthesized by the Petasis reaction. The structural analysis of the molecule is performed by FTIR, 1H, 13C NMR, and UV-Vis spectrometry and supported by the computational spectral studies. The structural properties and quantum chemical calculations are carried out using the density functional theory (DFT) at the B3LYP and B3PW91 level with a 6-311++G(d,p) basis set. The NMR data are calculated by means of the GIAO method; the TD–DFT method is used for UV-Vis spectroscopy. In addition, the electronic and structural properties (bond lengths, dihedral and bond angles), HOMO and LUMO energies, NLO analysis, thermodynamic parameters (rotation constants, entropy, thermal energy, thermal capacity), vibrational frequencies, electrostatic potential (MEP), excitation energies, Mulliken atomic charges, and oscillator strengths are investigated. The results show that the molecular properties of alkylaminophenol compounds are theoretically and experimentally compatible.






Similar content being viewed by others
REFERENCES
Y. Ulaş. Eur. J. Sci. Technol., 2019, 16, 242–247.
Y. Liu, L. Wang, Y. Siu, and J. Yu. Chin. J. Chem., 2010, 28, 2039–2044.
Y. Ulaş, A. İ. Özkan, and V. Tolan. Eur. J. Sci. Technol., 2019, 16, 701–706.
N. Takahashi, T. Ohba, T. Yamauchi, and K. Higashiyama. Bioorg. Med. Chem., 2006, 14, 6089–6096.
P. Doan, T. Nguyen, O. Yli-Harja, M. Kandhavelu, and N. Candeias. Eur. J. Pharm. Sci., 2017, 107, 208–216.
N. A. Petasis, A. Goodman, and I. A. Zavialov. Tetrahedron, 1997, 53, 16463–16470.
P. Wu, M. Givskov, and T. E. Nielsen. Chem. Rev., 2019, 119, 11245–11290.
L. Xu, S. Zhang, and P. Li. Chem. Soc. Rev., 2015, 44, 8848–8858.
N. J. McLean, H. Tye, and M. Whittaker. Tetrahedron Lett., 2004, 45, 993–995.
S. Reddy, B. Reddy, and P. Reddy. Tetrahedron Lett., 2015, 56, 4984–4989.
T. Koolmeister, M. Södergren, and M. Scobie. Tetrahedron Lett., 2002, 43, 5969–5970.
R. Hosseinzadeh, Z. Lasemi, M. Oloub, and M. Pooryousef. J. Iran Chem. Soc., 2017, 14, 347–355.
Y. Ulaş. Eur. J. Sci. Technol., 2020, 18, 574–582.
K. Subashini, R. Govindarajan, R. Surendran, K. Mukund, and S. Periandy. J. Mol. Struct., 2016, 1125, 576–591.
B. Kaboudin, A. Zangooei, F. Kazemi, and T. Yokomatsu. Tetrahedron Lett., 2018, 59, 1046–1049.
I. Kucuk, Y. Kaya, and A. A. Kaya. J. Mol. Struct., 2017, 1139, 308–318.
İ. Küçük and Y. Kaya. J. Innovative Sci. Eng., 2018, 2, 81–96.
N. Sundaraganesan, S. Ilakiamani, H. Saleem, P. M. Wojciechowski, and D. Michalska. Spectrochim. Acta, Part A, 2005, 61, 2995–3001.
A. J. L. Jesus, M. T. S. Rosado, I. Reva, R. Fausto, M. E. Eusebio, and J. S. Redinha. J. Phys. Chem. A, 2006, 110, 4169–4179.
J. P. P. L. Sherin, R. Hemamalini, S. Muthu, and A. A. Al-Saadi. Spectrochim. Acta, Part A, 2015, 146. 177–186.
Chemistry / Eds. R. Chang and K. A. Goldsby. New York, 2013.
K. S. Abdulov, N. U. Muloev, S. K. Tabarov, and M. K. Khodiev. J. Struct. Chem., 2020, 61, 510–514.
U.A. Soliman. J. Struct. Chem., 2020, 61(3), 400–418.
X. Yang, Z. H. Cao, Y. Zhou, F. Cheng, Z. W. Lin, Z. Ou, Y. Yuan, and Y. Y. Huang. Org. Lett., 2018, 20, 2585–2589.
M. E. Castro, M. J. Percino, V. M. Chapela, M. Ceron, G. Soriano-Moro, J. Lopez-Cruz, and F. J. Melendez. Int. J. Mol. Sci., 2013, 14, 4005–4029.
V. Arjunan, N. Puviarasan, and S. Mohan. Spectrochim. Acta, Part A, 2006, 64, 233–239.
K. Sharma, R. Melavanki, S. S. Patil, R. Kusanur, N. R. Patil, and V. M. Shelar. J. Mol. Struct., 2019, 1181, 474–487.
M. Govindarajan and M. Karabacak, Spectrochim. Acta, Part A, 2012, 96, 421–435.
M. Pandey, S. Muthu, and N. M. Nanje Gowda. J. Mol. Struct., 2017, 1130, 511–521.
Z. Gültekin, Z. Demircioğlu, W. Frey, and O. Büyükgüngör. J. Mol. Struct., 2020, 1199, 126970.
S. K. Pathak, N. G. Haress, A. A. El-Emam, R. Srivastava, O. Prasad, and L. Sinha. J. Mol. Struct., 2014, 1074, 457–466.
A. L. Varghese, I. Abraham, and M. George. Mater. Today Proc., 2019, 9, 92–96.
Funding
This study was supported with KUAP(F)-2016/5 project by Bursa Uludag University Scientific Research Projects Unit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflict of interests.
Additional information
Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 3, pp. 382-393.https://doi.org/10.26902/JSC_id69877
Rights and permissions
About this article
Cite this article
Ulaş, Y. THEORETICAL AND EXPERIMENTAL INVESTIGATION OF 2-((4-(HYDROXYMETHYL)PHENYL)(PYRROLIDIN-1-YL) METHYL)PHENOL: SYNTHESIS, SPECTROSCOPIC (FTIR, UV, NMR) STUDIES, AND NLO ANALYSIS. J Struct Chem 62, 356–368 (2021). https://doi.org/10.1134/S0022476621030021
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621030021