Abstract
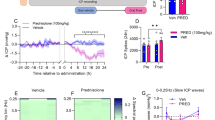
The administration of the adrenal hormone corticosterone by injection or subcutaneous implantation of its modified-release dosage forms is often used to model situations associated with the effects of various stressors on the brain. The present methodological study investigated the possibility of using Alzet osmotic pumps (OPs) with a capacity of 200 µL and a release rate of 1 µL/h, loaded with corticosterone solutions of 25, 50 and 100 mg/mL or a vehicle (dimethyl sulfoxide/polyethylene glycol-400). The objective was to determine the optimal concentration of corticosterone solution loaded into osmotic pumps to create an increased level of the hormone in the rat blood and brain regions. An increase in blood corticosterone levels was only observed on mg/mL (OP-100). Corticosterone accumulation in the frontal cortex, neocortex and hippocampus increased significantly by day 3 after implantation in the OP-100 group. Thus, OPs with a corticosterone solution in DMSO/PEG-400, at a hormone concentration of 100 mg/mL and release rate of 1 µL/h, can be used to create elevated corticosterone levels in the blood and brain regions of rats.


Similar content being viewed by others
REFERENCES
Gulyaeva NV (2019) Biochemical Mechanisms and Translational Relevance of Hippocampal Vulnerability to Distant Focal Brain Injury: The Price of Stress Response. Biochemistry (Mosc) 84: 1306–1328. https://doi.org/10.1134/S0006297919110087
Onufriev MV, Moiseeva YV, Zhanina MY, Lazareva NA, Gulyaeva NV (2021) A Comparative Study of Koizumi and Longa Methods of Intraluminal Filament Middle Cerebral Artery Occlusion in Rats: Early Corticosterone and Inflammatory Response in the Hippocampus and Frontal Cortex. Int J Mol Sci 22: 13544. https://doi.org/10.3390/ijms222413544
Komoltsev IG, Tret’yakova LV, Frankevich SO, Shirobokova NI, Volkova AA, Butuzov AV, Novikova MR, Kvichansky AA, Moiseeva YV, Onufriev MV, Bolshakov AP, Gulyaeva NV (2022) Neuroinflammatory Cytokine Response, Neuronal Death, and Microglial Proliferation in the Hippocampus of Rats During the Early Period After Lateral Fluid Percussion-Induced Traumatic Injury of the Neocortex. Mol Neurobiol 59: 1151–1167. https://doi.org/10.1007/s12035-021-02668-4
Bolshakov AP, Tret’yakova LV, Kvichansky AA, Gulyaeva NV (2021) Glucocorticoids: Dr. Jekyll and Mr. Hyde of Hippocampal Neuroinflammation. Biochemistry (Mosc) 86: 156–167. https://doi.org/10.1134/S0006297921020048
Buttgereit F, Burmester GR, Lipworth BJ (2005) Optimised glucocorticoid therapy: the sharpening of an old spear. Lancet 365: 801–803. https://doi.org/10.1016/S0140-6736(05)17989-6
Strehl C, Buttgereit F (2013) Optimized glucocorticoid therapy: teaching old drugs new tricks. Mol Cell Endocrinol 380: 32–40. https://doi.org/10.1016/j.mce.2013.01.026
Holmes MC, French KL, Seckl JR (1997) Dysregulation of diurnal rhythms of serotonin 5-HT2C and corticosteroid receptor gene expression in the hippocampus with food restriction and glucocorticoids. J Neurosci 17: 4056–4065. https://doi.org/10.1523/JNEUROSCI.17-11-04056.1997
Bush VL, Middlemiss DN, Marsden CA, Fone KC (2003) Implantation of a slow release corticosterone pellet induces long-term alterations in serotonergic neurochemistry in the rat brain. J Neuroendocrinol 15: 607–613. https://doi.org/10.1046/j.1365-2826.2003.01034.x
Mueller AD, Pollock MS, Lieblich SE, Epp JR, Galea LA, Mistlberger RE (2008) Sleep deprivation can inhibit adult hippocampal neurogenesis independent of adrenal stress hormones. Am J Physiol Regul Integr Comp Physiol 294: R1693–R1703. https://doi.org/10.1152/ajpregu.00858.2007
Coll AP, Challis BG, Lopez M, Piper S, Yeo GS, O’Rahilly S (2005) Proopiomelanocortin deficient mice are hypersensitive to the adverse metabolic effects of glucocorticoids. Diabetes 54: 2269–2276. https://doi.org/10.2337/diabetes.54.8.2269
Stone EA, Lin Y (2007) An anti-immobility effect of exogenous corticosterone in mice. Eur J Pharmacol 580: 135–142. https://doi.org/10.1016/j.ejphar.2007.10.045
Man MS, Young AH, McAllister-Williams RH (2002) Corticosterone modulation of somatodendritic 5-HT1A receptor function in mice. J Psychopharmacol 16: 245–252. https://doi.org/10.1177/026988110201600310
Yau SY, Li A, Zhang ED, Christie BR, Xu A, Lee TM, So KF (2014) Sustained running in rats administered corticosterone prevents the development of depressive behaviors and enhances hippocampal neurogenesis and synaptic plasticity without increasing neurotrophic factor levels. Cell Transplant 23: 481–492. https://doi.org/10.3727/096368914X678490
Nuber UA, Kriaucionis S, Roloff TC, Guy J, Selfridge J, Steinhoff C (2005) Up-regulation of glucocorticoid-regulated genes in a mouse model of Rett syndrome. Hum Mol Genet 14: 2247–2256. https://doi.org/10.1093/hmg/ddi229
Herrmann M, Henneicke H, Street J, Modzelewski J, Kalak R, Buttgereit F, Dunstan CR, Zhou H, Seibel MJ (2009) The challenge of continuous exogenous glucocorticoid administration in mice. Steroids 74: 245–249. https://doi.org/10.1016/j.steroids.2008.11.009
Claflin DI, Schmidt KD, Vallandingham ZD, Kraszpulski M, Hennessy MB (2017) Influence of postnatal glucocorticoids on hippocampal-dependent learning varies with elevation patterns and administration methods. Neurobiol Learn Mem143: 77–87. https://doi.org/10.1016/j.nlm.2017.05.010
Leal-Galicia P, Sánchez-Torres M, Meraz-Ríos M (2019) Cholesterol or Fat Rich Diets Accelerate Natural Age-Decline on Adult Hippocampal Neurogenesis and Have an Impact in Memory and Like-Anxiety Behavior. Advanc Biosci Biotechnol 10: 331–345. https://doi.org/10.4236/abb.2019.1010026
Herrmann M, Henneicke H, Street J, Modzelewski J, Kalak R, Buttgereit F, Dunstan CR, Zhou H, Seibel MJ (2009) The challenge of continuous exogenous glucocorticoid administration in mice. Steroids 74: 245–249. https://doi.org/10.1016/j.steroids.2008.11.009
Liu HH, Payne HR, Wang B, Brady ST (2006) Gender differences in response of hippocampus to chronic glucocorticoid stress: role of glutamate receptors. J Neurosci Res 83: 775–786. https://doi.org/10.1002/jnr.20782
Callaghan BL, Richardson R (2014) Early emergence of adult-like fear renewal in the developing rat after chronic corticosterone treatment of the dam or the pups. Behav Neurosci 128: 594–602. https://doi.org/10.1037/bne0000009
Claflin DI, Greenfield LR, Hennessy MB (2014) Modest elevation of corticosterone in preweanling rats impairs subsequent trace eyeblink conditioning during the juvenile period. Behav Brain Res 258: 19–26. https://doi.org/10.1016/j.bbr.2013.10.008
Funding
This work was supported by the Russian Science Foundation, grant no. 20-65-47029.
Author information
Authors and Affiliations
Contributions
Conceptualization and experimental design (N.V.G., M.V.O.); data collection and treatment (Yu.V.M.); writing and editing the manuscript (M.V.O., N.V.G.).
Corresponding authors
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 11, pp.1542–1550https://doi.org/10.31857/S0869813922110085.
Rights and permissions
About this article
Cite this article
Onufriev, M.V., Moiseeva, Y.V. & Gulyaeva, N.V. Modeling of Hypercorticosteronemia in Rats Using Osmotic Pumps. J Evol Biochem Phys 58, 1987–1993 (2022). https://doi.org/10.1134/S0022093022060266
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022060266