Abstract
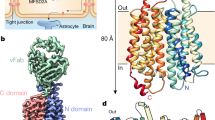
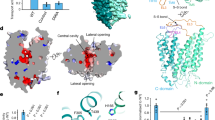
The protein Mfsd2a (Major Facilitator Superfamily Domain Containing 2a), a specific Na+-dependent transporter of lysophosphatidylcholine (lysoPC) esterified by omega-3 polyunsaturated fatty acids (PUFA), is selectively expressed in the endothelium of brain capillaries. This review summarizes current concepts on the molecular mechanisms of its functioning in endothelial cells and the role of transported lipids in the inhibition of caveolin-dependent transcytosis and maintaining a low permeability of the blood–brain barrier (BBB). A special focus is on the Mfsd2a-mediated lysoPC transfer as it is a major route of entry into the brain and retina for docosahexaenoic acid and other omega-3 PUFA essential for the normal development and functioning of the CNS and visual system. The protective role of Mfsd2a in various CNS neuropathologies is also discussed, as well as the prospects for developing Mfsd2a-based therapeutic strategies to increase the bioavailability of essential PUFA and to deliver the drugs that do not penetrate the BBB to brain tissue.


Similar content being viewed by others
REFERENCES
Hawkins BT, Davis TP (2005) The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57(2):173–185. https://doi.org/10.1124/pr.57.2.4
Salmina AB, Komleva YK, Malinovskaya NA, Morgun AV, Teplyashina EA, Lopatina OL, Gorina YV, Kharitonova EV, Khilazheva ED, Shuvaev AN (2021) Blood–brain Barrier Breakdown in Stress and Neurodegeneration: Biochemical Mechanisms and New Models for Translational Research. Biochemistry (Mosc) 86(6): 746–760. https://doi.org/10.1134/S0006297921060122
Kaya M, Ahishali B (2021) Basic physiology of the blood–brain barrier in health and disease: a brief overview. Tissue Barriers 9(1):1840913. https://doi.org/10.1080/21688370.2020.1840913
Brightman MW, Reese TS (1969) Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol 40(3):648–677. https://doi.org/10.1083/jcb.40.3.648
Reese TS, Karnovsky MJ (1967) Fine structural localization of a blood–brain barrier to exogenous peroxidase. J Cell Biol 34(1):207–217. https://doi.org/10.1083/jcb.34.1.207
Ayloo S, Gu C (2019) Transcytosis at the blood–brain barrier. Curr Opin Neurobiol 57:32–38. https://doi.org/10.1016/j.conb.2018.12.014
Yan N (2015) Structural Biology of the Major Facilitator Superfamily Transporters. Annu Rev Biophys 44:257–283. https://doi.org/10.1146/annurev-biophys-060414-033901
Reddy VS, Shlykov MA, Castillo R, Sun EI, Saier MH Jr (2012) The major facilitator superfamily (MFS) revisited. FEBS J 279(11):2022–2035. https://doi.org/10.1111/j.1742-4658.2012.08588.x
Angers M, Uldry M, Kong D, Gimble JM, Jetten AM (2008) Mfsd2a encodes a novel major facilitator superfamily domain-containing protein highly induced in brown adipose tissue during fasting and adaptive thermogenesis. Biochem J 416(3):347–355. https://doi.org/10.1042/BJ20080165
Cater RJ, Chua GL, Erramilli SK, Keener JE, Choy BC, Tokarz P, Chin CF, Quek DQY, Kloss B, Pepe JG, Parisi G, Wong BH, Clarke OB, Marty MT, Kossiakoff AA, Khelashvili G, Silver DL, Mancia F (2021) Structural basis of omega-3 fatty acid transport across the blood–brain barrier. Nature 595(7866):315–319. https://doi.org/10.1038/s41586-021-03650-9
Berger JH, Charron MJ, Silver DL (2012) Major facilitator superfamily domain-containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism. PLoS One 7(11): e50629. https://doi.org/10.1371/journal.pone.0050629
Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C (2014) Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature 509(7501):507–511. https://doi.org/10.1038/nature13324
Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, Silver DL (2014) Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509(7501):503–506. https://doi.org/10.1038/nature13241
Alakbarzade V, Hameed A, Quek DQ, Chioza BA, Baple EL, Cazenave-Gassiot A, Nguyen LN, Wenk MR, Ahmad AQ, Sreekantan-Nair A, Weedon MN, Rich P, Patton MA, Warner TT, Silver DL, Crosby AH (2015) A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet 47(7):814–817. https://doi.org/10.1038/ng.3313
Guemez-Gamboa A, Nguyen LN, Yang H, Zaki MS, Kara M, Ben-Omran T, Akizu N, Rosti RO, Rosti B, Scott E, Schroth J, Copeland B, Vaux KK, Cazenave-Gassiot A, Quek DQ, Wong BH, Tan BC, Wenk MR, Gunel M, Gabriel S, Chi NC, Silver DL, Gleeson JG (2015) Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet 47(7):809–813. https://doi.org/10.1038/ng.3311
Bazinet RP, Laye S (2014) Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci 15(12):771–785. https://doi.org/10.1038/nrn3820
Parnova R (2021) GPR40/FFA1 Free Fatty Acid Receptors and Their Functional Role. Neuroscience and Behavioral Physiology 51(2):256–264. https://doi.org/10.1007/s11055-021-01064-8
Crupi R, Marino A, Cuzzocrea S (2013) n-3 fatty acids: role in neurogenesis and neuroplasticity. Curr Med Chem 20(24):2953–2963. https://doi.org/10.2174/09298673113209990140
Lagarde M, Bernoud N, Brossard N, Lemaitre-Delaunay D, Thies F, Croset M, Lecerf J (2001) Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J Mol Neurosci 16(2–3):201–204; discussion 215–221. https://doi.org/10.1385/JMN:16:2-3:201
Thies F, Delachambre MC, Bentejac M, Lagarde M, Lecerf J (1992) Unsaturated fatty acids esterified in 2-acyl-l-lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. J Neurochem 59(3):1110–1116. https://doi.org/10.1111/j.1471-4159.1992.tb08353.x
Thies F, Pillon C, Moliere P, Lagarde M, Lecerf J (1994) Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Am J Physiol 267(5 Pt 2):R1273–R1279. https://doi.org/10.1152/ajpregu.1994.267.5.R1273
Sugasini D, Yalagala PCR, Goggin A, Tai LM, Subbaiah PV (2019) Enrichment of brain docosahexaenoic acid (DHA) is highly dependent upon the molecular carrier of dietary DHA: lysophosphatidylcholine is more efficient than either phosphatidylcholine or triacylglycerol. J Nutr Biochem 74:108231. https://doi.org/10.1016/j.jnutbio.2019.108231
Sugasini D, Thomas R, Yalagala PCR, Tai LM, Subbaiah PV (2017) Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci Rep 7(1):11263. https://doi.org/10.1038/s41598-017-11766-0
Chouinard-Watkins R, Lacombe RJS, Bazinet RP (2018) Mechanisms regulating brain docosahexaenoic acid uptake: what is the recent evidence? Curr Opin Clin Nutr Metab Care 21(2): 71–77. https://doi.org/10.1097/MCO.0000000000000440
Martins JG (2009) EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr 28(5):525–542. https://doi.org/10.1080/07315724.2009.10719785
Yalagala PCR, Sugasini D, Dasarathi S, Pahan K, Subbaiah PV (2019) Dietary lysophosphatidylcholine-EPA enriches both EPA and DHA in the brain: potential treatment for depression. J Lipid Res 60(3):566–578. https://doi.org/10.1194/jlr.M090464
Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C (2017) Blood–brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 94(3):581–594 e5. https://doi.org/10.1016/j.neuron.2017.03.043
Parton RG, Tillu VA, Collins BM (2018) Caveolae. Curr Biol 28(8):R402–R405. https://doi.org/10.1016/j.cub.2017.11.075
Wassall SR, Stillwell W (2008) Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem Phys Lipids 153(1):57–63. https://doi.org/10.1016/j.chemphyslip.2008.02.010
Li Q, Zhang Q, Wang M, Liu F, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J (2007) Docosahexaenoic acid affects endothelial nitric oxide synthase in caveolae. Arch Biochem Biophys 466(2): 250–259. https://doi.org/10.1016/j.abb.2007.06.023
Ma DW, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, Chapkin RS (2004) n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J 18(9):1040–1052. https://doi.org/10.1096/fj.03-1430fje
Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J (2007) Eicosapentaenoic acid modifies lipid composition in caveolae and induces translocation of endothelial nitric oxide synthase. Biochimie 89(1):169–177. https://doi.org/10.1016/j.biochi.2006.10.009
Turk HF, Chapkin RS (2013) Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 88(1):43–47. https://doi.org/10.1016/j.plefa.2012.03.008
Shaikh SR (2012) Biophysical and biochemical mechanisms by which dietary N-3 polyunsaturated fatty acids from fish oil disrupt membrane lipid rafts. J Nutr Biochem 23(2):101–105. https://doi.org/10.1016/j.jnutbio.2011.07.001
Eser Ocak P, Ocak U, Sherchan P, Zhang JH, Tang J (2020) Insights into major facilitator superfamily domain-containing protein-2a (Mfsd2a) in physiology and pathophysiology. What do we know so far? J Neurosci Res 98(1):29–41. https://doi.org/10.1002/jnr.24327
Cui Y, Wang Y, Song X, Ning H, Zhang Y, Teng Y, Wang J, Yang X (2021) Brain endothelial PTEN/AKT/NEDD4-2/MFSD2A axis regulates blood–brain barrier permeability. Cell Rep 36(1): 109327. https://doi.org/10.1016/j.celrep.2021.109327
Cui M, Gobel V, Zhang H (2022) Uncovering the “sphinx” of sphingosine 1-phosphate signalling: from cellular events to organ morphogenesis. Biol Rev Camb Philos Soc 97(1):251–272. https://doi.org/10.1111/brv.12798
Zhu D, Wang Y, Singh I, Bell RD, Deane R, Zhong Z, Sagare A, Winkler EA, Zlokovic BV (2010) Protein S controls hypoxic/ischemic blood–brain barrier disruption through the TAM receptor Tyro3 and sphingosine 1-phosphate receptor. Blood 115(23):4963–4972. https://doi.org/10.1182/blood-2010-01-262386
Wang Z, Zheng Y, Wang F, Zhong J, Zhao T, Xie Q, Zhu T, Ma F, Tang Q, Zhou B, Zhu J (2020) Mfsd2a and Spns2 are essential for sphingosine-1-phosphate transport in the formation and maintenance of the blood–brain barrier. Sci Adv 6(22):eaay8627. https://doi.org/10.1126/sciadv.aay8627
Huwiler A, Zangemeister-Wittke U (2018) The sphingosine 1-phosphate receptor modulator fingolimod as a therapeutic agent: Recent findings and new perspectives. Pharmacol Ther 185:34–49. https://doi.org/10.1016/j.pharmthera.2017.11.001
Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T, Sunden Y, Arai Y, Moriwaki K, Ishida J, Uemura A, Kiyonari H, Abe T, Fukamizu A, Hirashima M, Sawa H, Aoki J, Ishii M, Mochizuki N (2012) The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest 122(4):1416–1426. https://doi.org/10.1172/JCI60746
Cunha-Vaz JG, Shakib M, Ashton N (1966) Studies on the permeability of the blood-retinal barrier. I. On the existence, development, and site of a blood-retinal barrier. Br J Ophthalmol 50(8): 441–453. https://doi.org/10.1136/bjo.50.8.441
SanGiovanni JP, Chew EY (2005) The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res 24(1):87–138. https://doi.org/10.1016/j.preteyeres.2004.06.002
Fliesler SJ, Anderson RE (1983) Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res 22(2):79–131. https://doi.org/10.1016/0163-7827(83)90004-8
Aveldano MI, Sprecher H (1987) Very long chain (C24 to C36) polyenoic fatty acids of the n-3 and n-6 series in dipolyunsaturated phosphatidylcholines from bovine retina. J Biol Chem 262(3): 1180–1186.
Sugasini D, Yalagala PCR, Subbaiah PV (2020) Efficient Enrichment of Retinal DHA with Dietary Lysophosphatidylcholine-DHA: Potential Application for Retinopathies. Nutrients 12(10):3114. https://doi.org/10.3390/nu12103114
Lobanova ES, Schuhmann K, Finkelstein S, Lewis TR, Cady MA, Hao Y, Keuthan C, Ash JD, Burns ME, Shevchenko A, Arshavsky VY (2019) Disrupted Blood-Retina Lysophosphatidylcholine Transport Impairs Photoreceptor Health But Not Visual Signal Transduction. J Neurosci 39(49):9689–9701. https://doi.org/10.1523/jneurosci.1142-19.2019
Wong BH, Chan JP, Cazenave-Gassiot A, Poh RW, Foo JC, Galam DL, Ghosh S, Nguyen LN, Barathi VA, Yeo SW, Luu CD, Wenk MR, Silver DL (2016) Mfsd2a Is a Transporter for the Essential omega-3 Fatty Acid Docosahexaenoic Acid (DHA) in Eye and Is Important for Photoreceptor Cell Development. J Biol Chem 291(20):10501–10514. https://doi.org/10.1074/jbc.M116.721340
Wong BH, Silver DL (2020) Mfsd2a: A Physiologically Important Lysolipid Transporter in the Brain and Eye. Adv Exp Med Biol 1276:223–234. https://doi.org/10.1007/978-981-15-6082-8_14
Huang B, Li X (2021) The Role of Mfsd2a in Nervous System Diseases. Front Neurosci 15:730534. https://doi.org/10.3389/fnins.2021.730534
Yang YR, Xiong XY, Liu J, Wu LR, Zhong Q, Zhou K, Meng ZY, Liu L, Wang FX, Gong QW, Liao MF, Duan CM, Li J, Yang MH, Zhang Q, Gong CX, Yang QW (2017) Mfsd2a (Major Facilitator Superfamily Domain Containing 2a) Attenuates Intracerebral Hemorrhage-Induced Blood–Brain Barrier Disruption by Inhibiting Vesicular Transcytosis. J Am Heart Assoc 6(7):e005811. https://doi.org/10.1161/JAHA.117.005811
Jiao X, He P, Li Y, Fan Z, Si M, Xie Q, Chang X, Huang D (2015) The Role of Circulating Tight Junction Proteins in Evaluating Blood Brain Barrier Disruption following Intracranial Hemorrhage. Dis Markers 2015:860120. https://doi.org/10.1155/2015/860120
De Bock M, Van Haver V, Vandenbroucke RE, Decrock E, Wang N, Leybaert L (2016) Into rather unexplored terrain-transcellular transport across the blood–brain barrier. Glia 64(7):1097–1123. https://doi.org/10.1002/glia.22960
Zhao C, Ma J, Wang Z, Li H, Shen H, Li X, Chen G (2020) Mfsd2a Attenuates Blood–brain Barrier Disruption After Sub-arachnoid Hemorrhage by Inhibiting Caveolae-Mediated Transcellular Transport in Rats. Transl Stroke Res 11(5): 1012–1027. https://doi.org/10.1007/s12975-019-00775-y
Eser Ocak P, Ocak U, Sherchan P, Gamdzyk M, Tang J, Zhang JH (2020) Overexpression of Mfsd2a attenuates blood brain barrier dysfunction via Cav-1/Keap-1/Nrf-2/HO-1 pathway in a rat model of surgical brain injury. Exp Neurol 326: 113203. https://doi.org/10.1016/j.expneurol.2020.113203
Qu C, Song H, Shen J, Xu L, Li Y, Qu C, Li T, Zhang J (2020) Mfsd2a Reverses Spatial Learning and Memory Impairment Caused by Chronic Cerebral Hypoperfusion via Protection of the Blood–Brain Barrier. Front Neurosci 14:461. https://doi.org/10.3389/fnins.2020.00461
Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A, Vedin I, Vessby B, Wahlund LO, Palmblad J (2006) Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol 63(10):1402–1408. https://doi.org/10.1001/archneur.63.10.1402
Sanchez-Campillo M, Ruiz-Pastor MJ, Gazquez A, Marin-Munoz J, Noguera-Perea F, Ruiz-Alcaraz AJ, Manzanares-Sanchez S, Antunez C, Larque E (2019) Decreased Blood Level of MFSD2a as a Potential Biomarker of Alzheimer's Disease. Int J Mol Sci 21(1):70. https://doi.org/10.3390/ijms21010070
Semba RD (2020) Perspective: The Potential Role of Circulating Lysophosphatidylcholine in Neuroprotection against Alzheimer Disease. Adv Nutr 11(4):760–772. https://doi.org/10.1093/advances/nmaa024
Hachem M, Nacir H, Picq M, Belkouch M, Bernoud-Hubac N, Windust A, Meiller L, Sauvinet V, Feugier N, Lambert-Porcheron S, Laville M, Lagarde M (2020) Docosahexaenoic Acid (DHA) Bioavailability in Humans after Oral Intake of DHA-Containing Triacylglycerol or the Structured Phospholipid AceDoPC((R)). Nutrients 12(1):251. https://doi.org/10.3390/nu12010251
Hachem M, Geloen A, Van AL, Foumaux B, Fenart L, Gosselet F, Da Silva P, Breton G, Lagarde M, Picq M, Bernoud-Hubac N (2016) Efficient Docosahexaenoic Acid Uptake by the Brain from a Structured Phospholipid. Mol Neurobiol 53(5):3205–3215. https://doi.org/10.1007/s12035-015-9228-9
Ju X, Miao T, Chen H, Ni J, Han L (2021) Overcoming Mfsd2a-Mediated Low Transcytosis to Boost Nanoparticle Delivery to Brain for Chemotherapy of Brain Metastases. Adv Healthc Mater 10(9):e2001997. https://doi.org/10.1002/adhm.202001997
Wang JZ, Xiao N, Zhang YZ, Zhao CX, Guo XH, Lu LM (2016) Mfsd2a-based pharmacological strategies for drug delivery across the blood–brain barrier. Pharmacol Res 104:124–131. https://doi.org/10.1016/j.phrs.2015.12.024
Funding
This review article was implemented within the state assignment to the Sechenov Institute of Evolutionary Physiology and Biochemistry (Russian Academy of Sciences), no. 075-00776-19-02.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The author has no conflict of interest related to the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 5, pp. 547–561https://doi.org/10.31857/S0869813922050090.
Rights and permissions
About this article
Cite this article
Parnova, R.G. Critical Role of Endothelial Lysophosphatidylcholine Transporter Mfsd2a in Maintaining Blood–Brain Barrier Integrity and Delivering Omega 3 PUFA to the Brain. J Evol Biochem Phys 58, 742–754 (2022). https://doi.org/10.1134/S0022093022030103
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022030103