Abstract
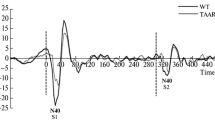
TAAR1 is known as a neuromodulator of monoaminergic systems, providing the negative regulation of dopaminergic and serotonergic neuronal activity. The TAAR1 ability to regulate monoaminergic systems determines its prominent role in psychiatric and neurological disorders. The present study is aimed to provide further evidence for the role of TAAR1 in mismatch negativity (MMN) generation. The electroencephalogram was recorded in freely moving TAAR1 knockout and wild type mice. As the MMN response reflects a combination of stimulus-specific adaptation (SSA) and deviance detection (DD) response, we compare standard and deviant stimuli to the multi-standard control. The difference observed between the high-adapted standard and low-adapted control stimuli together with the similar response to deviant and control stimuli suggest that the MMN-like response in wild type mice most likely reflects the SSA. On the other hand, TAAR1 knockout mice show no difference between standard, deviant, and control stimuli, probably indicating that the SSA to repetitive stimuli is impaired in TAAR-KO mice.

Similar content being viewed by others
REFERENCES
Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C (2001) Trace amines: Identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci 98:8966–8971. https://doi.org/10.1073/pnas.151105198
Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK (2001) Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol 60:1181–1188. https://doi.org/10.1124/mol.60.6.1181
Lindemann L, Meyer C, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, Bettler B, Wettstein J, Borroni E, Moreau J, Hoener M (2008) Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther 324:948–956. https://doi.org/10.1124/jpet.107.132647
Di Cara B, Maggio R, Aloisi G, Rivet JM, Lundius EG, Yoshitake T, Svenningsson P, Brocco M, Gobert A, de Groote L, Cistarelli L, Veiga S, de Montrion CD, Rodriguez M, Galizzi JP, Lockhart BP, Cogé F, Boutin JA, Vayer P, Verdouw PM, Groenink L, Millan MJ (2011) Genetic deletion of trace amine 1 receptors reveals their role in auto-inhibiting the actions of ecstasy (MDMA). J Neurosci 31:16928–16940. https://doi.org/10.1523/JNEUROSCI.2502-11.2011
Espinoza S, Lignani G, Caffino L, Maggi S, Sukhanov I, Leo D, Mus L, Emanuele M, Ronzitti G, Harmeier A, Medrihan L, Sotnikova TD, Chieregatti E, Hoener MC, Benfenati F, Tucci V, Fumagalli F, Gainetdinov RR (2015) TAAR1 modulates cortical glutamate NMDA receptor function. Neuropsychopharmacology 40:2217–2227. https://doi.org/10.1038/npp.2015.65
Pei Y, Asif-Malik A, Canales JJ (2016) Trace amines and the trace amine-associated receptor 1: Pharmacology, neurochemistry, and clinical implications. Front Neurosci 10:148. https://doi.org/10.3389/fnins.2016.00148
Berry MD, Gainetdinov RR, Hoener MC, Shahid M (2017) Pharmacology of human trace amine-associated receptors: Therapeutic opportunities and challenges. Pharmacol Ther 180:161–180. https://doi.org/10.1016/j.pharmthera.2017.07.002
John J, Kukshal P, Bhatia T, Chowdari KV, Nimgaonkar VL, Deshpande SN, Thelma BK (2017) Possible role of rare variants in trace amine associated receptor 1 in schizophrenia. Schizophr Res 189:190–195. https://doi.org/10.1016/j.schres.2017.02.020
Dodd S, F. Carvalho A, Puri BK, Maes M, Bortolasci CC, Morris G, Berk M (2021) Trace Amine-Associated Receptor 1 (TAAR1): A new drug target for psychiatry? Neurosci Biobehav Rev 120:537–541. https://doi.org/10.1016/j.neubiorev.2020.09.028.
Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky B, Seeman P, Branchek T, Gerald CP (2007) The Trace Amine 1 receptor knockout mouse: An animal model with relevance to schizophrenia. Genes, Brain Behav 6:628–639. https://doi.org/10.1111/j.1601-183X.2006.00292.x
Polyakova NV, Vinogradova EP, Aleksandrov AA, Gainetdinov RR (2018) Prepulse inhibition in the TAAR1 knockout mice. Ross Fiziol Zhurnal Im IM Sechenova 104:1098–1105. https://doi.org/10.1016/j.brainres.2011.04.010
Light GA, Swerdlow NR (2015) Future clinical uses of neurophysiological biomarkers to predict and monitor treatment response for schizophrenia. Ann N Y Acad Sci 1344:105–119. https://doi.org/10.1111/nyas.12730
Aleksandrov AA, Dmitrieva ES, Volnova AB, Knyazeva VM, Gainetdinov RR, Polyakova NV (2019) Effect of trace amine-associated receptor 1 agonist RO5263397 on sensory gating in mice. Neuroreport 30:1004–1007. https://doi.org/10.1097/wnr.0000000000001313
Aleksandrov AA, Knyazeva VM, Volnova AB, Dmitrieva ES, Polyakova NV, Gainetdinov RR (2019) Trace amine-associated receptor 1 agonist modulates mismatch negativity-like responses in mice. Front Pharmacol 10:470. https://doi.org/10.3389/fphar.2019.00470
Näätänen R, Kujala T, Winkler I (2011) Auditory processing that leads to conscious perception: A unique window to central auditory processing opened by the mismatch negativity and related responses. Psychophysiology 48:4–22. https://doi.org/10.1111/j.1469-8986.2010.01114.x
Näätänen R, Kähkönen S (2009) Central auditory dysfunction in schizophrenia as revealed by the mismatch negativity (MMN) and its magnetic equivalent MMNm: A review. Int J Neuropsychopharmacol 12:125–135 https://doi.org/10.1017/S1461145708009322
Javitt DC, Doneshka P, Zylberman I, Ritter W, Vaughan HG (1993) Impairment of early cortical processing in schizophrenia: An event-related potential confirmation study. Biol Psychiatry 33:513–519. https://doi.org/10.1016/0006-3223(93)90005-X
Michie PT (2001) What has MMN revealed about the auditory system in schizophrenia? Int J Psychophysiol 42:177–194. https://doi.org/10.1016/S0167-8760(01)00166-0
O’Reilly JA, Conway BA (2021) Classical and controlled auditory mismatch responses to multiple physical deviances in anaesthetised and conscious mice. Eur J Neurosci 53:1839–1854. https://doi.org/10.1111/ejn.15072
Ross JM, Hamm JP (2020) Cortical Microcircuit Mechanisms of Mismatch Negativity and Its Underlying Subcomponents. Front Neural Circuits 14:13. https://doi.org/10.3389/fncir.2020.00013
Bradaia A, Trube G, Stalder H, Norcross RD, Ozmen L, Wettstein JG, Pinard A, Buchy D, Gassmann M, Hoener MC, Bettler B (2009) The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc Natl Acad Sci 106:20081–20086. https://doi.org/10.1073/pnas.0906522106
Revel FG, Moreau J-L, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA, Metzler V, Chaboz S, Ozmen L, Trube G, Pouzet B, Bettler B, Caron MG, Wettstein JG, Hoener MC (2011) TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci 108:8485–8490. https://doi.org/10.1073/pnas.1103029108
Espinoza S, Salahpour A, Masri B, Sotnikova TD, Messa M, Barak LS, Caron MG, Gainetdinov RR (2011) Functional Interaction between Trace Amine-Associated Receptor 1 and Dopamine D2 Receptor. Mol Pharmacol 80:416–425. https://doi.org/10.1124/mol.111.073304
Revel FG, Moreau JL, Pouzet B, Mory R, Bradaia A, Buchy D, Metzler V, Chaboz S, Groebke Zbinden K, Galley G, Norcross RD, Tuerck D, Bruns A, Morairty SR, Kilduff TS, Wallace TL, Risterucci C, Wettstein JG, Hoener MC (2013) A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry 18:543–556. https://doi.org/10.1038/mp.2012.57
Thorn DA, Jing L, Qiu Y, Gancarz-Kausch AM, Galuska CM, Dietz DM, Zhang Y, Li JX (2014) Effects of the trace amine-associated receptor 1 agonist RO5263397 on abuse-related effects of cocaine in rats. Neuropsychopharmacology 39:2309–2316. https://doi.org/10.1038/npp.2014.91
Cotter R, Pei Y, Mus L, Harmeier A, Gainetdinov RR, Hoener MC, Canales JJ (2015) The trace amine-associated receptor 1 modulates methamphetamine’s neurochemical and behavioral effects. Front Neurosci 9:39. https://doi.org/10.3389/fnins.2015.00039
Liu JF, Seaman R, Siemian JN, Bhimani R, Johnson B, Zhang Y, Zhu Q, Hoener MC, Park J, Dietz DM, Li JX (2018) Role of trace amine-associated receptor 1 in nicotine’s behavioral and neurochemical effects. Neuropsychopharmacology 43:2435–2444. https://doi.org/10.1038/s41386-018-0017-9
Dedic N, Dworak H, Zeni C, Rutigliano G, Howes OD (2021) Therapeutic Potential of TAAR1 Agonists in Schizophrenia: Evidence from Preclinical Models and Clinical Studies. Int J Mol Sci 22:13185. https://doi.org/10.3390/ijms222413185
Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R (1998) Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull 24:189–202
Duque D, Malmierca MS, Caspary DM (2014) Modulation of stimulus-specific adaptation by GABAA receptor activation or blockade in the medial geniculate body of the anaesthetized rat. J Physiol 592:729–743. https://doi.org/10.1113/jphysiol.2013.261941
Nelken I (2014) Stimulus-specific adaptation and deviance detection in the auditory system: experiments and models. Biol Cybern 108:655–663. https://doi.org/10.1007/s00422-014-0585-7
Natan RG, Briguglio JJ, Mwilambwe-Tshilobo L, Jones SI, Aizenberg M, Goldberg EM, Geffen MN (2015) Complementary control of sensory adaptation by two types of cortical interneurons. Elife 4:e09868. https://doi.org/10.7554/eLife.09868
Malmierca MS, Anderson LA, Antunes FM (2015) The cortical modulation of stimulus-specific adaptation in the auditory midbrain and thalamus: A potential neuronal correlate for predictive coding. Front Syst Neurosci 9:19. https://doi.org/10.3389/fnsys.2015.00019
Leo D, Mus L, Espinoza S, Hoener MC, Sotnikova TD, Gainetdinov RR (2014) Taar1-mediated modulation of presynaptic dopaminergic neurotransmission: Role of D2 dopamine autoreceptors. Neuropharmacology 81:283–291. https://doi.org/10.1016/j.neuropharm.2014.02.007
Kähkönen S, Ahveninen J, Jääskeläinen IP, Kaakkola S, Näätänen R, Huttunen J, Pekkonen E (2001) Effects of haloperidol on selective attention: A combined whole-head MEG and high-resolution EEG study. Neuropsychopharmacology 25:498–504. https://doi.org/10.1016/S0893-133X(01)00255-X
Valdés-Baizabal C, Carbajal GV, Pérez-González D, Malmierca MS (2020) Dopamine modulates subcortical responses to surprising sounds. PLoS Biol 18:e3000744. https://doi.org/10.1371/journal.pbio.3000744
Ehlers CL, Wall TL, Chaplin RI (1991) Long latency event-related potentials in rats: Effects of dopaminergic and serotonergic depletions. Pharmacol Biochem Behav 38:789–793. https://doi.org/10.1016/0091-3057(91)90243-U
Ahveninen J, Kähkönen S, Pennanen S, Liesivuori J, Ilmoniemi RJ, Jääskeläinen IP (2002) Tryptophan depletion effects on EEG and MEG responses suggest serotonergic modulation of auditory involuntary attention in humans. Neuroimage 16:1052–1061. https://doi.org/10.1006/nimg.2002.1142
Kähkönen S, Mäkinen V, Jääskeläinen IP, Pennanen S, Liesivuori J, Ahveninen J (2005) Serotonergic modulation of mismatch negativity. Psychiatry Res—Neuroimaging 138:61–74. https://doi.org/10.1016/j.pscychresns.2004.09.006
Pan W, Lyu K, Zhang H, Li C, Chen P, Ying M, Chen F, Tang J (2020) Attenuation of auditory mismatch negativity in serotonin transporter knockout mice with anxiety-related behaviors. Behav Brain Res 379:112387. https://doi.org/10.1016/j.bbr.2019.11238741
Shen HW, Hagino Y, Kobayashi K, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch KP, Murphy DL, Hall FS, Uhl GR, Sora I (2004) Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology 29: 1790–1799. https://doi.org/10.1038/sj.npp.1300476
Funding
This work was supported by the Russian Science Foundation, project no. 22-25-00006.
Author information
Authors and Affiliations
Contributions
Conceptualization and experimental design (A.A.A., V.M.K.); data collection (E.S.D., N.V.P., Yu.A.S., L.N.S.); data processing (V.M.K., A.Yu.A.); writing and editing a manuscript (V.M.K., A.A.A., E.S.D.).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have neither evident not potential conflict of interest associated with the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2022, Vol. 58, No. 3, pp. 232–239https://doi.org/10.31857/S0044452922030044.
Rights and permissions
About this article
Cite this article
Knyazeva, V.M., Dmitrieva, E.S., Polyakova, N.V. et al. Stimulus Specific Adaptation Is Affected in Trace Amine-Associated Receptor 1 (TAAR1) Knockout Mice. J Evol Biochem Phys 58, 692–699 (2022). https://doi.org/10.1134/S0022093022030061
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022030061