Abstract
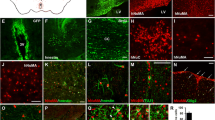
This work is based on the hypothesis of an impairment in neurogenesis during aging, which may be one of the causes of neurodegenerative diseases, including Alzheimer’s disease (AD). Uncompensated neuronal death leads to memory loss. There is an opinion that activation of endogenous neurogenesis or cell replacement therapy may be an effective treatment of AD. We used mesenchymal stromal cells (MSCs) isolated from the human umbilical cord Wharton’s jelly, which have a number of significant advantages over MSCs from other tissues. Human MSCs (hMSCs) were transplanted into the frontal cortex of 8–9-month-old female 5XFAD transgenic (Tg) mice, a model of the hereditary AD. To evaluate the effects of such transplantation, spatial memory was analyzed in parallel with morphofunctional characteristics of adult neurogenic niches—the subgranular zone (SGZ) of the hippocampal dentate gyrus and the subventricular zone (SVZ) of the lateral ventricles of the brain, as well as the brain areas responsible for learning and memory—the temporal cortex and CA1/CA3 fields of the hippocampus, using immunohistochemistry for markers of cell proliferation (BrdU) and neuronal differentiation (nestin, doublecortin, β-3 tubulin, NeuN, MAP2, GFAP). 5XFAD Tg mice were characterized by an impaired SGZ/SVZ ratio of proliferative activity and a reduced neuronal density in the cortex and hippocampus. The positive effect of hMSC treatment on memory and neuronal as well as glial density in the temporal cortex and hippocampal regions manifested itself two months after transplantation. By that time, hMSCs were detected in the brain of Tg mice only. Both in Tg and non-transgenic (nTg) mice treated with hMSCs, the density of BrdU+ cells was increased in the adult neurogenic niches, however only in the hippocampus of Tg mice, there was a reduced number of amyloid plaques and apoptotic cells, as well as an increased density of synaptophysin-immunopositive (SyP+) cells. Thus, the positive effect of hMSCs was manifested in the hippocampus of Tg mice, the structure remote from the frontal cortex, i.e. the transplantation site. Overall, our data indicate a paracrine effect of hMSCs, as well as the validity of the chimeric model and the promising use of MSC therapy in the treatment of AD.







Similar content being viewed by others
REFERENCES
Caselli RJ, Beach TG, Knopman DS, Graff-Radford NR (2017) Alzheimer Disease: Scientific Breakthroughs and Translational Challenges. Mayo Clin Proc 92(6):978-994. https://doi.org/10.1016/j.mayocp.2017.02.011
Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M (2019) Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomedicine 19(14):5541-5554. https://doi.org/10.2147/IJN.S200490
Loera-Valencia R, Piras A, Ismail MAM, Manchanda S, Eyjolfsdottir H, Saido TC. Johansson J, Eriksdotter M, Winblad B, Nilsson P (2018) Targeting Alzheimer’s disease with gene and cell therapies. J Int Med 284(1):2-36. https://doi.org/10.1111/joim.12759
Sadigh-Eteghad S, Sabermarouf B, Majdi A, Taleb M, Farhoudi M, Mahmoudi Jl (2015) Amyloid-beta: a crucial factor in Alzheimer’s disease. Med Princ Pract 24(1):1–10. https://doi.org/10.1159/000369101
Lane CA, Hardy J, Schott JM (2018) Alzheimer’s disease. Eur J Neurol 25(1):59–70. https://doi.org/10.1111/ene.13439
Winner B, Kohl Z, Gage FH (2011) Neurodegenerative disease and adult neurogenesis. Eur J Neurosci. 33(6):1139–1151. https://doi.org/10.1111/j.1460-9568.2011.07613.x
Liang X, Ding Y, Zhang Y, Tse HF, Lian Q (2014) Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant 23(9):1045–1059. https://doi.org/10.3727/096368913x667709
Vaes JEG, Vink MA, de Theije CGM, Hoebeek FE, Benders MJNL, Nijboer CHA (2019) The Potential of Stem Cell Therapy to Repair White Matter Injury in Preterm Infants: Lessons Learned From Experimental Models. Front Physiol 10: 540. https://doi.org/10.3389/fphys.2019.00540
Noureddini M, Bagheri-Mohammadi S (2021) Adult Hippocampal Neurogenesis and Alzheimer’s Disease: Novel Application of Mesenchymal Stem Cells and their Role in Hippocampal Neurogenesis. Int J Mol Cell Med 10(1):1–10. https://doi.org/10.22088/IJMCM.BUMS.10.1.1
Vitek MP, Araujo JA, Fossel M, Greenberg BD, Howel GR, Sukoff Rizzo SJ, Seyfried NT, Tenner AJ, Territo PR, Windisch M, Bain LJ, Ross AR, Carrillo MC, Lamb BT, Edelmayer RM (2021) Translational animal models for Alzheimer’s disease: An Alzheimer’s Association Business Consortium Think Tank. Alzheimers Dement (NY) 6(1):e12114. https://doi.org/10.1002/trc2.12114
Paton MCB, McDonald CA, Allison BJ, Fahey MC, Jenkin G, Miller SL (2017) Perinatal brain injury as a consequence of preterm birth and intrauterine inflammation: designing targeted stem cell therapies. Front Neurosci 11:200. https://doi.org/10.3389/fnins.2017.00200
De Miguel MP, Fuentes-Julian S, Blazquez-Martinez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F (2012) Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med 12(5):574–591. https://doi.org/10.2174/156652412800619950
Jacobs SA, Roobrouck VD, Verfaillie CM, Van Gool SW (2013) Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol 91(1):32–39. https://doi.org/10.1038/icb.2012.64
Kobolak J, Dinnyes A, Memic A, Khademhosseini A, Mobasheri A (2016) Mesenchymal stem cells: identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods 99:62–68. https://doi.org/10.1016/j.ymeth.2015.09.016
Volkman R, Offen D (2017) Concise review: mesenchymal stem cells in neurodegenerative diseases. Stem Cells 35:1867–1880. https://doi.org/10.1002/stem.2651
Frausin S, Viventi S, Verga Falzacappa L, Quattromani MJ, Leanza G, Tommasini A, Valencic E (2015) Wharton’s jelly derived mesenchymal stromal cells: Biological properties, induction of neuronal phenotype and current applications in neurodegeneration research. Acta Histochem 117(4–5):329–338. https://doi.org/10.1016/j.acthis.2015.02.005
Donders R, Bogie JFJ, Ravanidis S, Gervois P, Vanheusden M, Marée R, Schrynemackers M, Smeets HJM, Pinxteren J, Gijbels K, Walbers S, Mays RW, Deans R, Van Den Bosch L, Stinissen P, Lambrichts I, Gyselaers W, Hellings N (2018) Human Wharton’s Jelly-Derived Stem Cells Display a Distinct Immunomodulatory and Proregenerative Transcriptional Signature Compared to Bone Marrow-Derived Stem Cells. Stem Cells 27(2):65–84. https://doi.org/10.1089/scd.2017.0029
Shi J, Zhao YC, Niu ZF, Fan HJ, Hou SK, Guo XQ, Sang L, Lv Q (2021) Mesenchymal stem cell-derived small extracellular vesicles in the treatment of human diseases: progress and prospect. World J Stem Cells 13(1):49–63. https://doi.org/10.4252/wjsc.v13.i1.49
Wang X, Thomsen P (2021) Mesenchymal stem cell-derived small extracellular vesicles and bone regeneration. Basic Clin Pharmacol Toxicol 128:18–36. https://doi.org/10.1111/bcpt.13478
Zhdanova DY, Poltavtseva RA, Svirshchevskaya EV, Bobkova NV (2021) Effect of Intranasal Administration of Multipotent Mesenchymal Stromal Cell Exosomes on Memory of Mice in Alzheimer’s Disease Model. Bull Exp Biol Med 170(4): 575–582. https://doi.org/10.1007/s10517-021-05109-3
Venugopal CKS, Rai KS, Pinnelli VB, Kutty BM, Dhanushkodi A (2018) Neuroprotection by Human Dental Pulp Mesenchymal Stem Cells: From Billions to Nano. Curr Gene Ther 18(5):307–323. https://doi.org/10.2174/1566523218666180913152615
Mead B, Logan A, Berry M, Leadbeater W, Scheven BA (2014) Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One 9(10):e109305. https://doi.org/10.1371/journal.pone.0109305
Labrador-Velandia S, Alonso-Alonso ML, Di Lauro S, García-Gutierrez MT, Srivastava GK, Pastor JC, Fernandez-Bueno I (2019) Mesenchymal stem cells provide paracrine neuroprotective resources that delay degeneration of co-cultured organotypic neuroretinal cultures. Exp Eye Res 185:107671. https://doi.org/10.1016/j.exer.2019.05.011
Peng C, Li Y, Lu L, Zhu J, Li H, Hu J (2019) Efficient One-Step Induction of Human Umbilical Cord-Derived Mesenchymal Stem Cells (UC-MSCs) Produces MSC-Derived Neurospheres (MSC-NS) with Unique Transcriptional Profile and Enhanced Neurogenic and Angiogenic Secretomes. Stem Cells Int 2019:9208173. https://doi.org/10.1155/2019/9208173
Lee HJ, Lee JK, Lee H, Shin J-W, Carter JE, Sakamoto T, Kyung Jin H, Bae J-s (2010) The therapeutic potential of human umbilical cord blood-derived mesenchymal stem cells in Alzheimer’s disease. Neurosci Lett 481(1):30–35. https://doi.org/10.1016/j.neulet.2010.06.045
Park H-J, Shin JY, Lee BR, Kim HO, Lee PH (2012) Mesenchymal stem cells augment neurogenesis in the subventricular zone and enhance differentiation of neural precursor cells into dopaminergic neurons in the Substantia Nigra of a Parkinsonian model. Cell Transplant 21:1629–1640. https://doi.org/10.3727/096368912X640556
Kim J-Y, Kim DH, Kim JH, Kim J-Y, Lee D, Jeon HB, Kwon S-J, Kim SM, Yoo YJ, Lee EH, Choi SJ, Seo SW, Lee JI, Na DL, Yang YS, Oh W, Chang JW (2012) Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-β plaques. Cell Death Differ 19:680–691. https://doi.org/10.1038/cdd.2011.140
Shin JY, Park HJ, Kim HN, Oh SH, Bae J-S, Ha H-J, Lee PH (2014) Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer disease models. Autophagy 10:32–44. https://doi.org/10.4161/auto.26508
Oh SH, Kim HN, Park H-J, Shin JY, Lee PH (2015) Mesenchymal stem cells increase hippocampal neurogenesis and neuronal differentiation by enhancing the Wnt signaling pathway in an Alzheimer’s disease model. Cell Transpl 24:1097–1109. https://doi.org/10.3727/096368914X679237
Kalinina YA, Gilerovich EG, Korzhevskij DE (2019) Astrocytes and their participation in the mechanisms of therapeutic action of multipotent mesenchymal stromal cells in ischemic brain damage. Genes and Cells 14 (1): 33–40. https://doi.org/10.23868/201903004
Bobkova NV, Poltavtseva RA, Samokhin AN, Sukhikh GT (2013) Therapeutic effect of mesenchymal multipotent stromal cells on memory in animals with Alzheimer-type neurodegeneration. Bull Exp Biol Med 156(1):119–121. https://doi.org/10.1007/s10517-013-2293-z
Poltavtseva RA, Samokhin AN, Bobkova NV, Alexandrova MA, Sukhikh GT (2020) Effect of Transplantation of Neural Stem and Progenitor Cells on Memory in Animals with Alzheimer’s Type Neurodegeneration. Bull Exp Biol Med 168(4):589–596. https://doi.org/10.1007/s10517-020-04758-0
Arsent’yeva YEV, Polyakova DI (2021) A modern view of the use of cell technologies to stimulate reparative neurogenesis. Scientific Review. Med Sci 2: 16–24. (In Russ).
Franklin KBJ, Paxinos G (2008) The mouse brain in stereotaxic coordinates. Elsevier, Amsterdam.
Nemecek S, Subrtova D (1995) Cerebrospinal fluid dissemination of fetal neural isografts in brain of adult rats. Sb Ved Pr Lek Fak Karlovy Univer Hradci Kralove 38(1):5–9.
Werner M, Von Wasielewski R, Komminoth P (1996) Antigen retrival, signal amplification and intensification in immunohistochemistry. Histochem Cell Biol 105(4):253–260. https://doi.org/10.1007/BF01463928
Zhu M, Li W, Guo J, Lu Y, Dong X, Lin B, Chen Y, Zhang X, Li M (2016) Alpha fetoprotein antagonises benzyl isothiocyanate inhibition of the malignant behaviors of hepatocellular carcinoma cells. Oncotarget 7(46):75749–75762. https://doi.org/10.18632/oncotarget.12407
Wang W, Zhu M, Xu Z, Li W, Dong X, Chen Y, Lin B, Li M (2019) Ropivacaine promotes apoptosis of hepatocellular carcinoma cells through damaging mitochondria and activating caspase-3 activity. Biol Res 52(1):36. https://doi.org/10.1186/s40659-019-0242-7
Yi S, Liu G, Wu Y, Liang Q, Li L (2020) Baicalein suppresses the growth of the human thyroid cancer cells by inducing mitotic catastrophe, apoptosis and autophagy via NF-kB signalling pathway. J BUON 25(1):389–394.
Du WJ, Chi Y, Yang ZX, Li ZJ, Cui JJ, Song BQ, Li X, Yang SG, Han ZB, Han ZC (2016) Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res & Therapy 7(1):1–1. https://doi.org/10.1186/s13287-016-0418-9
Borner C (2003) The Bcl2 protein family: Sensors and checkpoints for life-or-death decisions. Mol Immunol 39:615–647. https://doi.org/10.1016/s0161-5890(02)00252-3
Lewandowska U, Szewczyk K, Owczarek K, Hrabec Z, Podsędek A, Koziołkiewicz M, Hrabec E (2013) Flavanols from Evening Primrose (Oenothera paradoxa) Defatted Seeds Inhibit Prostate Cells Invasiveness and Cause Changes in Bcl-2/Bax mRNA Ratio. J Agricult and Food Chem 61(12):2987–2998. https://doi.org/10.1021/jf304269x
Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Vassar R (2006) Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci 26 (40):10129–10140. https://doi.org/10.1523/JNEUROSCI.1202-06.2006
Bobkova NV, Lyabin DN, Medvinskaya NI, Samokhin AN, Nekrasov PV, Nesterova IV, Ovchinnikov LP (2015) The Y-box binding protein 1 suppresses Alzheimer’s disease progression in two animal models. PLoS One 10 (9):e0138867. https://doi.org/10.1371/journal.pone.0138867
Lazarov O, Marr RA (2010) Neurogenesis and Alzheimer’s disease: at the crossroads. Exp Neurol 223(2):267–281. https://doi.org/10.1016/j.expneurol.2009.08.009
Bonds JA, Kuttner-Hirshler Y, Bartolotti N, Tobin MK, Pizzi M, Marr R, Lazarov O (2015) Presenilin-1 dependent neurogenesis regulates hippocampal learning and memory. PLoS One 10(6):e0131266. https://doi.org/10.1371/journal.pone.0131266
Baum LW (2005) Sex, hormones, and Alzheimer’s disease. J Gerontol Ser A: Biol Sci and Med Sci 60(6):736–743. https://doi.org/10.1093/gerona/60.6.736
Webber KM, Casadesus G, Perry G, Atwood CS, Bowen R, Smith MA (2005) Gender differences in Alzheimer disease: the role of luteinizing hormone in disease pathogenesis. Alzheimer Disease & Associated Disorders 19(2):95–99. https://doi.org/10.1097/01.wad.0000165512.90864.3f
Mielke MM, Vemuri P, Rocca WA (2014) Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol 6:37–48. https://doi.org/10.2147/CLEP.S37929
Rodriguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, Oddo S, Verkhratsky A (2008) Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s disease. PloS one 13;3(8):e2935. https://doi.org/10.1371/journal.pone.0002935
Hall AM, Roberson ED (2012) Mouse models of Alzheimer’s disease. Brain Res Bull 88(1):3–12. https://doi.org/10.1016/j.brainresbull.2011.11.017
Vehmas AK, Kawas CH, Stewart WF, Troncoso JC (2003) Immune reactive cells in senile plaques and cognitive decline in Alzheimer’s disease. Neurobiol Aging 24(2):321–331. https://doi.org/10.1016/S0197-4580(02)00090-8
Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D (1989) Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol 24(3):173–182. https://doi.org/10.1016/0165-5728(89)90115-X
Hartlage-Rübsamen M, Zeitschel U, Apelt J, Gärtner U, Franke H, Stahl T, Günther A, Schliebs R, Penkowa M, Bigl V, Roßner S (2003) Astrocytic expression of the Alzheimer’s disease β-secretase (BACE1) is stimulus-dependent. Glia 41(2):169–179. https://doi.org/10.1002/glia.10178
Koistinaho M, Lin S, Wu XI, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, Paul SM (2004) Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-β peptides. Nature Med 10(7):719–726. https://doi.org/10.1038/nm1058
von Bartheld CS, Bahney J, Herculano-Houzel S (2016) The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Compar Neurol 524(18):3865–3895. https://doi.org/10.1002/cne.24040
Douaud G, Groves AR, Tamnes CK, Westlye LT, Duff EP, Engvig A, Walhovd KB, James A, Gass A, Monsch AU, Matthews PM (2014) A common brain network links development, aging, and vulnerability to disease. Proc Natl Acad Sci USA 111(49):17648–17653. https://doi.org/10.1073/pnas.1410378111
Evgen’ev M, Bobkova N, Krasnov G, Garbuz D, Funikov S, Kudryavtseva A, Kulikov A, Samokhin A, Maltsev A, Nesterova I (2019) The Effect of Human HSP70 Administration on a Mouse Model of Alzheimer’s Disease Strongly Depends on Transgenicity and Age. J Alzheimers Dis 67(4):1391–1404. https://doi.org/10.3233/JAD-180987
Coppé JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol: Mechanisms of Disease 5:99–118. https://doi.org/10.1146/annurev-pathol-121808-102144
Li D, Tang J, Xu H, Fan X, Bai Y, Yang L (2008) Decreased hippocampal cell proliferation correlates with increased expression of BMP4 in the APPswe/PS1ΔE9 mouse model of Alzheimer’s disease. Hippocampus 18(7):692–698. https://doi.org/10.1002/hipo.20428
Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ (2006) Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Compar Neurol 495(1):70–83. https://doi.org/10.1002/cne.20840
Taniuchi N, Niidome T, Goto Y, Akaike A, Kihara T, Sugimoto H (2007) Decreased proliferation of hippocampal progenitor cells in APPswe/PS1dE9 transgenic mice. Neuroreport 18(17):1801–1805. https://doi.org/10.1097/WNR.0b013e3282f1c9e9
Demars M, Hu YS, Gadadhar A, Lazarov O (2010) Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J Neurosci Res 88(10):2103–2117. https://doi.org/10.1002/jnr.22387
Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R (2018) Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22(4):589–599. https://doi.org/10.1016/j.stem.2018.03.015
Zhang R, Cai Y, Xiao R, Zhong H, Li X, Guo L, Xu H, Fan X (2019) Human amniotic epithelial cell transplantation promotes neurogenesis and ameliorates social deficits in BTBR mice. Stem Cell Res & Therapy 10(1):1–3. https://doi.org/10.1186/s13287-019-1267-0
Shi Q, Chowdhury S, Ma R, Le KX, Hong S, Caldarone BJ, Stevens B, Lemere CA (2017) Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci Translat Med 9(392):aaf6295. https://doi.org/10.1126/scitranslmed.aaf6295
Novoselova EG, Bobkova NV, Sinotova OA, Ogai VB, Glushkova OV, Medvinskaya NI, Samokhin AN (2003) The immune state of bulbectomized mice. Dokl Biol Sci 393:505–507. https://doi.org/10.1023/b:dobs.0000010308.59629.c0
Vercelli A, Mereuta OM, Garbossa D, Muraca G, Mareschi K, Rustichelli D, Ferrero I, Mazzini L, Madon E, Fagioli F (2008) Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Disease 31(3):395–405. https://doi.org/10.1016/j.nbd.2008.05.016
Björklund A, Lindvall O (2000) Cell replacement therapies for central nervous system disorders. Nature Neurosci 3(6):537–544. https://doi.org/10.1038/75705
Aithal AP, Bairy LK, Seetharam RN (2021) Safety and therapeutic potential of human bone marrow-derived mesenchymal stromal cells in regenerative medicine. Stem Cell Investig 8:10. https://doi.org/10.21037/sci-2020-036
Ko HR, Ahn SY, Chang YS, Hwang I, Yun T, Sung DK, Sung SI, Park WS, Ahn JY (2018) Human UCB-MSCs treatment upon intraventricular hemorrhage contributes to attenuate hippocampal neuron loss and circuit damage through BDNF-CREB signaling. Stem Cell Res & Therapy 9(1):1–5. https://doi.org/10.1186/s13287-018-1052-5
Varela-Nallar L, Inestrosa NC (2013) Wnt signaling in the regulation of adult hippocampal neurogenesis. Front Cell Neurosci 7:100. https://doi.org/10.3389/fncel.2013.00100
Xu C, Fu F, Li X, Zhang S (2017) Mesenchymal stem cells maintain the microenvironment of central nervous system by regulating the polarization of macrophages/microglia after traumatic brain injury. Int J Neurosci 127(12):1124–1135. https://doi.org/10.1080/00207454.2017.1325884
Papazian I, Kyrargyri V, Evangelidou M, Voulgari-Kokota A, Probert L (2018) Mesenchymal stem cell protection of neurons against glutamate excitotoxicity involves reduction of NMDA-triggered calcium responses and surface GluR1, and is partly mediated by TNF. Int J Mol Sci 19(3):651. https://doi.org/10.3390/ijms1903065
Reuss B, Halbach OV (2003) Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res 313(2):139–157. https://doi.org/10.1007/s00441-003-0756-7
Isele NB, Lee HS, Landshamer S, Straube A, Padovan CS, Plesnila N, Culmsee C (2007) Bone marrow stromal cells mediate protection through stimulation of PI3-K/Akt and MAPK signaling in neurons. Neurochem Int 50(1):243–250. https://doi.org/10.1016/j.neuint.2006.08.007
Park HJ, Shin JY, Kim HN, Oh SH, Song SK, Lee PH (2015) Mesenchymal stem cells stabilize the blood–brain barrier through regulation of astrocytes. Stem Cell Res & Therapy 6(1):1–2. https://doi.org/10.1186/s13287-015-0180-4
Park HJ, Lee PH, Bang OY, Lee G, Ahn YH (2008) Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson’s disease. J Neurochem 107(1):141–151. https://doi.org/10.1111/j.1471-4159.2008.05589.x
Park HJ, Bang G, Lee BR, Kim HO, Lee PH (2011) Neuroprotective effect of human mesenchymal stem cells in an animal model of double toxin induced multiple system atrophy parkinsonism. Cell Transplant 20:827–835. https://doi.org/10.3727/096368910X540630
Poltavtsev AM, Poltavtseva RA, Yushina MN, Volgina NE, Svirshchevskaya EV (2017) Cytokine Production in Mixed Cultures of Mesenchymal Stromal Cells from Wharton’s Jelly and Peripheral Blood Lymphocytes. Bull Exp Biol Med 163(1):169–175. https://doi.org/10.1007/s10517-017-3759-1
Poltavtseva RA, Bobkova NV, Zhdanova DYU, Svirshchevskaya EV, Sukhikh GT (2021) Alzheimer’s type neurodegeneration. possible correction of memory impairment with intravenous injection of exosomes. Biol Membr 38(5): 374–387. https://doi.org/10.31857/S0233475521050066
ACKNOWLEDGMENTS
The authors are grateful to P.V. Nekrasov for technical assistance in conducting experiments. The study was performed on the equipment of the Sector of Optical Microscopy and Spectrophotometry at the Pushchino Research Center for Biological Studies, Russian Academy of Sciences.
Funding
This work was supported by the Russian Science Foundation (project No. 18-15-00392).
Author information
Authors and Affiliations
Contributions
The idea and experimental design (B.N.V. and P.R.A.); data collection (C.A.V., Z.D.Y., K.V.I., M.N.I., P.R.A.) and processing (B.N.V., K.V.I., Z.D.Y., C.A.V., P.R.A.); cell isolation and culturing (P.R.A.), writing and editing a manuscript (B.N.V., Z.D.Y., K.V.I., P.R.A).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest, both evident and potential, that would be associated with the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 1, pp. 59–84https://doi.org/10.31857/S0869813922010046.
Rights and permissions
About this article
Cite this article
Chaplygina, A.V., Zhdanova, D.Y., Kovalev, V.I. et al. Cell Therapy as a Way to Correct Impaired Neurogenesis in the Adult Brain in a Model of Alzheimer’s Disease. J Evol Biochem Phys 58, 117–137 (2022). https://doi.org/10.1134/S0022093022010112
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022010112