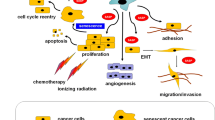
Abstract
Plasticity of tumor cells (multitude of molecular regulation pathways) allows them to evade cytocidal effects of chemo- and/or radiation therapy. Metabolic adaptation of the surviving cells is based on transcriptional reprogramming. Similarly to the process of natural cell aging, specific features of the survived tumor cells comprise the therapy-induced senescence phenotype. Tumor cells with this phenotype differ from the parental cells since they become less responsive to drugs and form aggressive progeny. Importance of the problem is explained by the general biological significance of transcriptional reprogramming as a mechanism of adaptation to stress, and by the emerging potential of its pharmacological targeting. In this review we analyze the mechanisms of regulation of the therapy-induced tumor cell senescence, as well as new drug combinations aimed to prevent this clinically unfavorable phenomenon.


Similar content being viewed by others
Abbreviations
- BIRC5 :
-
gene encoding survivin protein
- CDK:
-
cyclin-dependent protein kinase
- EMT:
-
epithelial–mesenchymal transition
- ROS:
-
reactive oxygen species
- SASP:
-
senescence-associated secretory phenotype
References
Kalyanaraman, B. (2020) Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: have we been barking up the wrong tree? Redox Biol., 29, 101394, https://doi.org/10.1016/j.redox.2019.101394.
White, S. C., Anderson, H., Jayson, G. C., Ashcroft, L., Ranson, M., and Thatcher, N. (2000) Randomised phase II study of cisplatin-etoposide versus infusional carboplatin in advanced non-small-cell lung cancer and mesothelioma, Ann. Oncol., 11, 201-206, https://doi.org/10.1023/a:1008328605413.
Cai, F., Luis, M. A. F., Lin, X., Wang, M., Cai, L., Cen, C., and Biskup, E. (2019) Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: Preventive strategies and treatment, Mol. Clin. Oncol., 11, 15-23, https://doi.org/10.3892/mco.2019.1854.
Te Poele, R. H., Okorokov, A. L., Jardine, L., Cummings, J., and Joel, S. P. (2002) DNA damage is able to induce senescence in tumor cells in vitro and in vivo, Cancer Res., 62, 1876-1883.
Demaria, M., O’Leary, M. N., Chang, J., Shao, L., Liu, S., Alimirah, F., Koenig, K., Le, C., Mitin, N., Deal, A. M., Alston, S., Academia, E. C., Kilmarx, S., Valdovinos, A., Wang, B., de Bruin, A., Kennedy, B. K., Melov, S., Zhou, D., Sharpless, N. E., et al. (2017) Cellular senescence promotes adverse effects of chemotherapy and cancer relapse, Cancer Discov., 7, 165-176, https://doi.org/10.1158/2159-8290.CD-16-0241.
Spallarossa, P., Altieri, P., Aloi, C., Garibaldi, S., Barisione, C., Ghigliotti, G., Fugazza, G., Barsotti, A., and Brunelli, C. (2009) Doxorubicin induces senescence or apoptosis in rat neonatal cardiomyocytes by regulating the expression levels of the telomere binding factors 1 and 2, Am. J. Physiol. Heart Circ. Physiol., 297, H2169-2181, https://doi.org/10.1152/ajpheart.00068.2009.
Probin, V., Wang, Y., Bai, A., and Zhou, D. (2006) Busulfan selectively induces cellular senescence but not apoptosis in WI38 fibroblasts via a p53-independent but extracellular signal-regulated kinase-p38 mitogen-activated protein kinase-dependent mechanism, J. Pharmacol. Exp. Ther., 319, 551-560, https://doi.org/10.1124/jpet.106.107771.
Seluanov, A., Gorbunova, V., Falcovitz, A., Sigal, A., Milyavsky, M., Zurer, I., Shohat, G., Goldfinger, N., and Rotter, V. (2001) Change of the death pathway in senescent human fibroblasts in response to DNA damage is caused by an inability to stabilize p53, Mol. Cell Biol., 21, 1552-1564, https://doi.org/10.1128/MCB.21.5.1552-1564.2001.
Soto-Gamez, A., Quax, W. J., and Demaria, M. (2019) Regulation of survival networks in senescent cells: from mechanisms to interventions, J. Mol. Biol., 431, 2629-2643, https://doi.org/10.1016/j.jmb.2019.05.036.
Chen, Z., Cao, K., Xia, Y., Li, Y., Hou, Y., Wang, L., Li, L., Chang, L., and Li, W. (2019) Cellular senescence in ionizing radiation (review), Oncol. Rep., 42, 883-894, https://doi.org/10.3892/or.2019.7209.
Fitsiou, E., Soto-Gamez, A., and Demaria, M. (2022) Biological functions of therapy-induced senescence in cancer, Semin. Cancer Biol., 81, 5-13, https://doi.org/10.1016/j.semcancer.2021.03.021.
Mijit, M., Caracciolo, V., Melillo, A., Amicarelli, F., and Giordano, A. (2020) Role of p53 in the regulation of cellular senescence, Biomolecules, 10, 420, https://doi.org/10.3390/biom10030420.
Rohnalter, V., Roth, K., Finkernagel, F., Adhikary, T., Obert, J., Dorzweiler, K., Bensberg, M., Muller-Brusselbach, S., and Muller, R. (2015) A multi-stage process including transient polyploidization and EMT precedes the emergence of chemoresistent ovarian carcinoma cells with a dedifferentiated and pro-inflammatory secretory phenotype, Oncotarget, 6, 40005-40025, https://doi.org/10.18632/oncotarget.5552.
Saleh, T., Bloukh, S., Carpenter, V. J., Alwohoush, E., Bakeer, J., Darwish, S., Azab, B., and Gewirtz, D. A. (2020) Therapy-induced senescence: An “old” friend becomes the enemy, Cancers (Basel), 12, 822, https://doi.org/10.3390/cancers12040822.
Shtil, A. A. (2002) Emergence of multidrug resistance in leukemia cells during chemotherapy: mechanisms and prevention, J. Hematother. Stem Cell Res., 11, 231-241, https://doi.org/10.1089/152581602753658439.
Mosteiro, L., Pantoja, C., Alcazar, N., Marion, R. M., Chondronasiou, D., Rovira, M., Fernandez-Marcos, P. J., Munoz-Martin, M., Blanco-Aparicio, C., Pastor, J., Gomez-Lopez, G., De Martino, A., Blasco, M. A., Abad, M., and Serrano, M. (2016) Tissue damage and senescence provide critical signals for cellular reprogramming in vivo, Science, 354, aaf4445, https://doi.org/10.1126/science.aaf4445.
Gabellini, C., Castellini, L., Trisciuoglio, D., Kracht, M., Zupi, G., and Del Bufalo, D. (2008) Involvement of nuclear factor-kappa B in bcl-xL-induced interleukin 8 expression in glioblastoma, J. Neurochem., 107, 871-882, https://doi.org/10.1111/j.1471-4159.2008.05661.x.
Fan, Y., Mao, R., and Yang, J. (2013) NF-kappaB and STAT3 signaling pathways collaboratively link inflammation to cancer, Protein Cell, 4, 176-185, https://doi.org/10.1007/s13238-013-2084-3.
Wang, B., Kohli, J., and Demaria, M. (2020) Senescent cells in cancer therapy: friends or foes? Trends Cancer, 6, 838-857, https://doi.org/10.1016/j.trecan.2020.05.004.
Calcinotto, A., Kohli, J., Zagato, E., Pellegrini, L., Demaria, M., and Alimonti, A. (2019) Cellular senescence: aging, cancer, and injury, Physiol. Rev., 99, 1047-1078, https://doi.org/10.1152/physrev.00020.2018.
Calcinotto, A., and Alimonti, A. (2017) Aging tumour cells to cure cancer: “pro-senescence” therapy for cancer, Swiss Med. Wkly, 147, w14367, https://doi.org/10.4414/smw.2017.14367.
Milanovic, M., Fan, D. N. Y., Belenki, D., Dabritz, J. H. M., Zhao, Z., Yu, Y., Dorr, J. R., Dimitrova, L., Lenze, D., Monteiro Barbosa, I. A., Mendoza-Parra, M. A., Kanashova, T., Metzner, M., Pardon, K., Reimann, M., Trumpp, A., Dorken, B., Zuber, J., Gronemeyer, H., Hummel, M., et al. (2018) Senescence-associated reprogramming promotes cancer stemness, Nature, 553, 96-100, https://doi.org/10.1038/nature25167.
Karabicici, M., Alptekin, S., Firtina Karagonlar, Z., and Erdal, E. (2021) Doxorubicin-induced senescence promotes stemness and tumorigenicity in EpCAM-/CD133-nonstem cell population in hepatocellular carcinoma cell line, HuH-7, Mol. Oncol., 15, 2185-2202, https://doi.org/10.1002/1878-0261.12916.
Pacifico, F., Badolati, N., Mellone, S., Stornaiuolo, M., Leonardi, A., and Crescenzi, E. (2021) Glutamine promotes escape from therapy-induced senescence in tumor cells, Aging (Albany NY), 13, 20962-20991, https://doi.org/10.18632/aging.203495.
Dorr, J. R., Yu, Y., Milanovic, M., Beuster, G., Zasada, C., Dabritz, J. H., Lisec, J., Lenze, D., Gerhardt, A., Schleicher, K., Kratzat, S., Purfurst, B., Walenta, S., Mueller-Klieser, W., Graler, M., Hummel, M., Keller, U., Buck, A. K., Dorken, B., Willmitzer, L., et al. (2013) Synthetic lethal metabolic targeting of cellular senescence in cancer therapy, Nature, 501, 421-425, https://doi.org/10.1038/nature12437.
Ratushnyy, A. Y., Rudimova, Y. V., and Buravkova, L. B. (2020) Replicative senescence and expression of autophagy genes in mesenchymal stromal cells, Biochemistry (Moscow), 85, 1169-1177, https://doi.org/10.1134/S0006297920100053.
Yang, N., and Sen, P. (2018) The senescent cell epigenome, Aging (Albany NY), 10, 3590-3609, https://doi.org/10.18632/aging.101617.
Gonzalez-Meljem, J. M., Apps, J. R., Fraser, H. C., and Martinez-Barbera, J. P. (2018) Paracrine roles of cellular senescence in promoting tumourigenesis, Br. J. Cancer, 118, 1283-1288, https://doi.org/10.1038/s41416-018-0066-1.
Zhao, Z., Dong, Q., Liu, X., Wei, L., Liu, L., Li, Y., and Wang, X. (2020) Dynamic transcriptome profiling in DNA damage-induced cellular senescence and transient cell-cycle arrest, Genomics, 112, 1309-1317, https://doi.org/10.1016/j.ygeno.2019.07.020.
Gonzalez-Gualda, E., Baker, A. G., Fruk, L., and Munoz-Espin, D. (2021) A guide to assessing cellular senescence in vitro and in vivo, FEBS J., 288, 56-80, https://doi.org/10.1111/febs.15570.
Davan-Wetton, C. S. A., Pessolano, E., Perretti, M., and Montero-Melendez, T. (2021) Senescence under appraisal: hopes and challenges revisited, Cell. Mol. Life Sci., 78, 3333-3354, https://doi.org/10.1007/s00018-020-03746-x.
Hernandez-Segura, A., Nehme, J., and Demaria, M. (2018) Hallmarks of cellular senescence, Trends Cell Biol., 28, 436-453, https://doi.org/10.1016/j.tcb.2018.02.001.
Dimri, G. P., Lee, X., Basile, G., Acosta, M., Scott, G., Roskelley, C., Medrano, E. E., Linskens, M., Rubelj, I., Pereira-Smith, O. et al. (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo, Proc. Natl. Acad. Sci. USA, 92, 9363-9367, https://doi.org/10.1073/pnas.92.20.9363.
Cahu, J., and Sola, B. (2013) A sensitive method to quantify senescent cancer cells, J. Vis. Exp., 2, 50494, https://doi.org/10.3791/50494.
Mikula-Pietrasik, J., Niklas, A., Uruski, P., Tykarski, A., and Ksiazek, K. (2020) Mechanisms and significance of therapy-induced and spontaneous senescence of cancer cells, Cell. Mol. Life Sci., 77, 213-229, https://doi.org/10.1007/s00018-019-03261-8.
Dikovskaya, D., Cole, J. J., Mason, S. M., Nixon, C., Karim, S. A., McGarry, L., Clark, W., Hewitt, R. N., Sammons, M. A., Zhu, J., Athineos, D., Leach, J. D., Marchesi, F., van Tuyn, J., Tait, S. W., Brock, C., Morton, J. P., Wu, H., Berger, S. L., Blyth, K., et al. (2015) Mitotic stress is an integral part of the oncogene-induced senescence program that promotes multinucleation and cell cycle arrest, Cell Rep., 12, 1483-1496, https://doi.org/10.1016/j.celrep.2015.07.055.
Matias, I., Diniz, L. P., Damico, I. V., Araujo, A. P. B., Neves, L. D. S., Vargas, G., Leite, R. E. P., Suemoto, C. K., Nitrini, R., Jacob-Filho, W., Grinberg, L. T., Hol, E. M., Middeldorp, J., and Gomes, F. C. A. (2022) Loss of lamin-B1 and defective nuclear morphology are hallmarks of astrocyte senescence in vitro and in the aging human hippocampus, Aging Cell, 21, e13521, https://doi.org/10.1111/acel.13521.
Freund, A., Laberge, R. M., Demaria, M., and Campisi, J. (2012) Lamin B1 loss is a senescence-associated biomarker, Mol. Biol. Cell, 23, 2066-2075, https://doi.org/10.1091/mbc.E11-10-0884.
Liao, C., Xiao, Y., and Liu, L. (2020) The dynamic process and its dual effects on tumors of therapy-induced senescence, Cancer Manag. Res., 12, 13553-13566, https://doi.org/10.2147/CMAR.S285083.
Mosieniak, G., Sliwinska, M. A., Alster, O., Strzeszewska, A., Sunderland, P., Piechota, M., Was, H., and Sikora, E. (2015) Polyploidy formation in doxorubicin-treated cancer cells can favor escape from senescence, Neoplasia, 17, 882-893, https://doi.org/10.1016/j.neo.2015.11.008.
Czarnecka-Herok, J., Sliwinska, M. A., Herok, M., Targonska, A., Strzeszewska-Potyrala, A., Bojko, A., Wolny, A., Mosieniak, G., and Sikora, E. (2022) Therapy-induced senescent/polyploid cancer cells undergo atypical divisions associated with altered expression of meiosis, spermatogenesis and EMT genes, Int. J. Mol. Sci., 23, 8288, https://doi.org/10.3390/ijms23158288.
Kumari, R., and Jat, P. (2021) Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype, Front. Cell Dev. Biol., 9, 645593, https://doi.org/10.3389/fcell.2021.645593.
Shtutman, M., Chang, B. D., Schools, G. P., and Broude, E. V. (2017) Cellular model of p21-induced senescence, Methods Mol. Biol., 1534, 31-39, https://doi.org/10.1007/978-1-4939-6670-7_3.
Romanov, V. S., Pospelov, V. A., and Pospelova, T. V. (2012) Cyclin-dependent kinase inhibitor p21(Waf1): contemporary view on its role in senescence and oncogenesis, Biochemistry (Moscow), 77, 575-584, https://doi.org/10.1134/S000629791206003X.
Gire, V., and Dulic, V. (2015) Senescence from G2 arrest, revisited, Cell Cycle, 14, 297-304, https://doi.org/10.1080/15384101.2014.1000134.
Moein, S., Adibi, R., da Silva Meirelles, L., Nardi, N. B., and Gheisari, Y. (2020) Cancer regeneration: Polyploid cells are the key drivers of tumor progression, Biochim. Biophys. Acta Rev. Cancer, 1874, 188408, https://doi.org/10.1016/j.bbcan.2020.188408.
Wang, Q., Wu, P. C., Dong, D. Z., Ivanova, I., Chu, E., Zeliadt, S., Vesselle, H., and Wu, D. Y. (2013) Polyploidy road to therapy-induced cellular senescence and escape, Int. J. Cancer, 132, 1505-1515, https://doi.org/10.1002/ijc.27810.
Song, Y., Zhao, Y., Deng, Z., Zhao, R., and Huang, Q. (2021) Stress-induced polyploid giant cancer cells: unique way of formation and non-negligible characteristics, Front. Oncol., 11, 724781, https://doi.org/10.3389/fonc.2021.724781.
Niklander, S. E., Lambert, D. W., and Hunter, K. D. (2021) Senescent cells in cancer: wanted or unwanted citizens, Cells, 10, 3315, https://doi.org/10.3390/cells10123315.
Coppe, J. P., Patil, C. K., Rodier, F., Sun, Y., Munoz, D. P., Goldstein, J., Nelson, P. S., Desprez, P. Y., and Campisi, J. (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor, PLoS Biol., 6, 2853-2868, https://doi.org/10.1371/journal.pbio.0060301.
Salminen, A., Kauppinen, A., and Kaarniranta, K. (2012) Emerging role of NF-kappaB signaling in the induction of senescence-associated secretory phenotype (SASP), Cell Signal., 24, 835-845, https://doi.org/10.1016/j.cellsig.2011.12.006.
Takasugi, M., Yoshida, Y., Hara, E., and Ohtani, N. (2022) The role of cellular senescence and SASP in tumour microenvironment, FEBS J., https://doi.org/10.1111/febs.16381.
Fisher, D. T., Appenheimer, M. M., and Evans, S. S. (2014) The two faces of IL-6 in the tumor microenvironment, Semin. Immunol., 26, 38-47, https://doi.org/10.1016/j.smim.2014.01.008.
Junaid, M., Lee, A., Kim, J., Park, T. J., and Lim, S. B. (2022) Transcriptional heterogeneity of cellular senescence in cancer, Mol. Cells, 45, 610-619, https://doi.org/10.14348/molcells.2022.0036.
Lau, L., and David, G. (2019) Pro- and anti-tumorigenic functions of the senescence-associated secretory phenotype, Expert Opin. Ther. Targets, 23, 1041-1051, https://doi.org/10.1080/14728222.2019.1565658.
Faget, D. V., Ren, Q., and Stewart, S. A. (2019) Unmasking senescence: context-dependent effects of SASP in cancer, Nat. Rev. Cancer, 19, 439-453, https://doi.org/10.1038/s41568-019-0156-2.
Yang, L., Fang, J., and Chen, J. (2017) Tumor cell senescence response produces aggressive variants, Cell Death Discov., 3, 17049, https://doi.org/10.1038/cddiscovery.2017.49.
Bojko, A., Staniak, K., Czarnecka-Herok, J., Sunderland, P., Dudkowska, M., Sliwinska, M. A., Salmina, K., and Sikora, E. (2020) Improved autophagic flux in escapers from doxorubicin-induced senescence/polyploidy of breast cancer cells, Int. J. Mol. Sci., 21, 6084, https://doi.org/10.3390/ijms21176084.
Puig, P. E., Guilly, M. N., Bouchot, A., Droin, N., Cathelin, D., Bouyer, F., Favier, L., Ghiringhelli, F., Kroemer, G., Solary, E., Martin, F., and Chauffert, B. (2008) Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy, Cell Biol. Int., 32, 1031-1043, https://doi.org/10.1016/j.cellbi.2008.04.021.
Song, Z., Pan, Y., Ling, G., Wang, S., Huang, M., Jiang, X., and Ke, Y. (2017) Escape of U251 glioma cells from temozolomide-induced senescence was modulated by CDK1/survivin signaling, Am. J. Transl. Res., 9, 2163-2180.
Luyties, O., and Taatjes, D. J. (2022) The Mediator kinase module: an interface between cell signaling and transcription, Trends Biochem. Sci., 47, 314-327, https://doi.org/10.1016/j.tibs.2022.01.002.
Richter, W. F., Nayak, S., Iwasa, J., and Taatjes, D. J. (2022) The Mediator complex as a master regulator of transcription by RNA polymerase II, Nat. Rev. Mol. Cell Biol., 23, 732-749, https://doi.org/10.1038/s41580-022-00498-3.
Saleh, T., Tyutyunyk-Massey, L., Murray, G. F., Alotaibi, M. R., Kawale, A. S., Elsayed, Z., Henderson, S. C., Yakovlev, V., Elmore, L. W., Toor, A., Harada, H., Reed, J., Landry, J. W., and Gewirtz, D. A. (2019) Tumor cell escape from therapy-induced senescence, Biochem. Pharmacol., 162, 202-212, https://doi.org/10.1016/j.bcp.2018.12.013.
Pluquet, O., Abbadie, C., and Coqueret, O. (2019) Connecting cancer relapse with senescence, Cancer Lett., 463, 50-58, https://doi.org/10.1016/j.canlet.2019.08.004.
Elmore, L. W., Di, X., Dumur, C., Holt, S. E., and Gewirtz, D. A. (2005) Evasion of a single-step, chemotherapy-induced senescence in breast cancer cells: implications for treatment response, Clin. Cancer Res., 11, 2637-2643, https://doi.org/10.1158/1078-0432.CCR-04-1462.
Jonchere, B., Vetillard, A., Toutain, B., Lam, D., Bernard, A. C., Henry, C., De Carne Trecesson, S., Gamelin, E., Juin, P., Guette, C., and Coqueret, O. (2015) Irinotecan treatment and senescence failure promote the emergence of more transformed and invasive cells that depend on anti-apoptotic Mcl-1, Oncotarget, 6, 409-426, https://doi.org/10.18632/oncotarget.2774.
Roberson, R. S., Kussick, S. J., Vallieres, E., Chen, S. Y., and Wu, D. Y. (2005) Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers, Cancer Res., 65, 2795-2803, https://doi.org/10.1158/0008-5472.CAN-04-1270.
Ashraf, H. M., Moser, J., and Spencer, S. L. (2019) Senescence evasion in chemotherapy: a sweet spot for p21, Cell, 178, 267-269, https://doi.org/10.1016/j.cell.2019.06.025.
Roninson, I. B. (2003) Tumor cell senescence in cancer treatment, Cancer Res., 63, 2705-2715.
Hsu, C. H., Altschuler, S. J., and Wu, L. F. (2019) Patterns of early p21 dynamics determine proliferation-senescence cell fate after chemotherapy, Cell, 178, 361-373.e312, https://doi.org/10.1016/j.cell.2019.05.041.
Olszewska, A., Borkowska, A., Granica, M., Karolczak, J., Zglinicki, B., Kieda, C., and Was, H. (2021) Escape from cisplatin-induced senescence of hypoxic lung cancer cells can be overcome by hydroxychloroquine, Front. Oncol., 11, 738385, https://doi.org/10.3389/fonc.2021.738385.
Guillon, J., Petit, C., Moreau, M., Toutain, B., Henry, C., Roche, H., Bonichon-Lamichhane, N., Salmon, J. P., Lemonnier, J., Campone, M., Verriele, V., Lelievre, E., Guette, C., and Coqueret, O. (2019) Regulation of senescence escape by TSP1 and CD47 following chemotherapy treatment, Cell Death Dis., 10, 199, https://doi.org/10.1038/s41419-019-1406-7.
Guillon, J., Coquelet, H., Leman, G., Toutain, B., Petit, C., Henry, C., Boissard, A., Guette, C., and Coqueret, O. (2021) tRNA biogenesis and specific aminoacyl-tRNA synthetases regulate senescence stability under the control of mTOR, PLoS Genet., 17, e1009953, https://doi.org/10.1371/journal.pgen.1009953.
De Carne Trecesson, S., Guillemin, Y., Belanger, A., Bernard, A. C., Preisser, L., Ravon, E., Gamelin, E., Juin, P., Barre, B., and Coqueret, O. (2011) Escape from p21-mediated oncogene-induced senescence leads to cell dedifferentiation and dependence on anti-apoptotic Bcl-xL and MCL1 proteins, J. Biol. Chem., 286, 12825-12838, https://doi.org/10.1074/jbc.M110.186437.
Yew, T. L., Chiu, F. Y., Tsai, C. C., Chen, H. L., Lee, W. P., Chen, Y. J., Chang, M. C., and Hung, S. C. (2011) Knockdown of p21(Cip1/Waf1) enhances proliferation, the expression of stemness markers, and osteogenic potential in human mesenchymal stem cells, Aging Cell, 10, 349-361, https://doi.org/10.1111/j.1474-9726.2011.00676.x.
Yosef, R., Pilpel, N., Papismadov, N., Gal, H., Ovadya, Y., Vadai, E., Miller, S., Porat, Z., Ben-Dor, S., and Krizhanovsky, V. (2017) p21 maintains senescent cell viability under persistent DNA damage response by restraining JNK and caspase signaling, EMBO J., 36, 2280-2295, https://doi.org/10.15252/embj.201695553.
Vetillard, A., Jonchere, B., Moreau, M., Toutain, B., Henry, C., Fontanel, S., Bernard, A. C., Campone, M., Guette, C., and Coqueret, O. (2015) Akt inhibition improves irinotecan treatment and prevents cell emergence by switching the senescence response to apoptosis, Oncotarget, 6, 43342-43362, https://doi.org/10.18632/oncotarget.6126.
Martinez, L. A., Yang, J., Vazquez, E. S., Rodriguez-Vargas Mdel, C., Olive, M., Hsieh, J. T., Logothetis, C. J., and Navone, N. M. (2002) p21 modulates threshold of apoptosis induced by DNA-damage and growth factor withdrawal in prostate cancer cells, Carcinogenesis, 23, 1289-1296, https://doi.org/10.1093/carcin/23.8.1289.
Zhang, Y., Gao, Y., Zhang, G., Huang, S., Dong, Z., Kong, C., Su, D., Du, J., Zhu, S., Liang, Q., Zhang, J., Lu, J., and Huang, B. (2011) DNMT3a plays a role in switches between doxorubicin-induced senescence and apoptosis of colorectal cancer cells, Int. J. Cancer, 128, 551-561, https://doi.org/10.1002/ijc.25365.
Sohn, D., Essmann, F., Schulze-Osthoff, K., and Janicke, R. U. (2006) p21 blocks irradiation-induced apoptosis downstream of mitochondria by inhibition of cyclin-dependent kinase-mediated caspase-9 activation, Cancer Res., 66, 11254-11262, https://doi.org/10.1158/0008-5472.CAN-06-1569.
Kuang, Y., Kang, J., Li, H., Liu, B., Zhao, X., Li, L., Jin, X., and Li, Q. (2021) Multiple functions of p21 in cancer radiotherapy, J. Cancer Res. Clin. Oncol., 147, 987-1006, https://doi.org/10.1007/s00432-021-03529-2.
Doktorova, H., Hrabeta, J., Khalil, M. A., and Eckschlager, T. (2015) Hypoxia-induced chemoresistance in cancer cells: the role of not only HIF-1, Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub., 159, 166-177, https://doi.org/10.5507/bp.2015.025.
Jing, X., Yang, F., Shao, C., Wei, K., Xie, M., Shen, H., and Shu, Y. (2019) Role of hypoxia in cancer therapy by regulating the tumor microenvironment, Mol. Cancer, 18, 157, https://doi.org/10.1186/s12943-019-1089-9.
White-Gilbertson, S., and Voelkel-Johnson, C. (2020) Giants and monsters: Unexpected characters in the story of cancer recurrence, Adv. Cancer Res., 148, 201-232, https://doi.org/10.1016/bs.acr.2020.03.001.
Sabisz, M., and Skladanowski, A. (2009) Cancer stem cells and escape from drug-induced premature senescence in human lung tumor cells: implications for drug resistance and in vitro drug screening models, Cell Cycle, 8, 3208-3217, https://doi.org/10.4161/cc.8.19.9758.
Sikora, E., Czarnecka-Herok, J., Bojko, A., and Sunderland, P. (2022) Therapy-induced polyploidization and senescence: Coincidence or interconnection? Semin. Cancer Biol., 81, 83-95, https://doi.org/10.1016/j.semcancer.2020.11.015.
Lin, K. C., Torga, G., Sun, Y., Axelrod, R., Pienta, K. J., Sturm, J. C., and Austin, R. H. (2019) The role of heterogeneous environment and docetaxel gradient in the emergence of polyploid, mesenchymal and resistant prostate cancer cells, Clin. Exp. Metastasis, 36, 97-108, https://doi.org/10.1007/s10585-019-09958-1.
Achuthan, S., Santhoshkumar, T. R., Prabhakar, J., Nair, S. A., and Pillai, M. R. (2011) Drug-induced senescence generates chemoresistant stemlike cells with low reactive oxygen species, J. Biol. Chem., 286, 37813-37829, https://doi.org/10.1074/jbc.M110.200675.
Niu, N., Zhang, J., Zhang, N., Mercado-Uribe, I., Tao, F., Han, Z., Pathak, S., Multani, A. S., Kuang, J., Yao, J., Bast, R. C., Sood, A. K., Hung, M. C., and Liu, J. (2016) Linking genomic reorganization to tumor initiation via the giant cell cycle, Oncogenesis, 5, e281, https://doi.org/10.1038/oncsis.2016.75.
Niu, N., Mercado-Uribe, I., and Liu, J. (2017) Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells, Oncogene, 36, 4887-4900, https://doi.org/10.1038/onc.2017.72.
Salmina, K., Bojko, A., Inashkina, I., Staniak, K., Dudkowska, M., Podlesniy, P., Rumnieks, F., Vainshelbaum, N. M., Pjanova, D., Sikora, E., and Erenpreisa, J. (2020) “Mitotic slippage” and extranuclear DNA in cancer chemoresistance: A focus on telomeres, Int. J. Mol. Sci., 21, 2779, https://doi.org/10.3390/ijms21082779.
Sikora, E., Mosieniak, G., and Sliwinska, M. A. (2016) Morphological and functional characteristic of senescent cancer cells, Curr. Drug Targets, 17, 377-387, https://doi.org/10.2174/1389450116666151019094724.
Was, H., Czarnecka, J., Kominek, A., Barszcz, K., Bernas, T., Piwocka, K., and Kaminska, B. (2018) Some chemotherapeutics-treated colon cancer cells display a specific phenotype being a combination of stem-like and senescent cell features, Cancer Biol. Ther., 19, 63-75, https://doi.org/10.1080/15384047.2017.1385675.
Diaz-Carballo, D., Saka, S., Klein, J., Rennkamp, T., Acikelli, A. H., Malak, S., Jastrow, H., Wennemuth, G., Tempfer, C., Schmitz, I., Tannapfel, A., and Strumberg, D. (2018) A distinct oncogenerative multinucleated cancer cell serves as a source of stemness and tumor heterogeneity, Cancer Res., 78, 2318-2331, https://doi.org/10.1158/0008-5472.CAN-17-1861.
Was, H., Barszcz, K., Czarnecka, J., Kowalczyk, A., Bernas, T., Uzarowska, E., Koza, P., Klejman, A., Piwocka, K., Kaminska, B., and Sikora, E. (2017) Bafilomycin A1 triggers proliferative potential of senescent cancer cells in vitro and in NOD/SCID mice, Oncotarget, 8, 9303-9322, https://doi.org/10.18632/oncotarget.14066.
Chen, J., Wei, H., Cheng, J., Xie, B., Wang, B., Yi, J., Tian, B., Liu, Z., Wang, F., and Zhang, Z. (2018) Characteristics of doxorubicin-selected multidrug-resistant human leukemia HL-60 cells with tolerance to arsenic trioxide and contribution of leukemia stem cells, Oncol. Lett., 15, 1255-1262, https://doi.org/10.3892/ol.2017.7353.
Zhou, H. M., Zhang, J. G., Zhang, X., and Li, Q. (2021) Targeting cancer stem cells for reversing therapy resistance: mechanism, signaling, and prospective agents, Signal. Transduct. Target Ther., 6, 62, https://doi.org/10.1038/s41392-020-00430-1.
Yuan, R., Liu, Q., Segeren, H. A., Yuniati, L., Guardavaccaro, D., Lebbink, R. J., Westendorp, B., and de Bruin, A. (2019) Cyclin F-dependent degradation of E2F7 is critical for DNA repair and G2-phase progression, EMBO J., 38, e101430, https://doi.org/10.15252/embj.2018101430.
Schulz, A., Meyer, F., Dubrovska, A., and Borgmann, K. (2019) Cancer stem cells and radioresistance: DNA repair and beyond, Cancers (Basel), 11, 862, https://doi.org/10.3390/cancers11060862.
Gold, A., Eini, L., Nissim-Rafinia, M., Viner, R., Ezer, S., Erez, K., Aqaqe, N., Hanania, R., Milyavsky, M., Meshorer, E., and Goldberg, M. (2019) Spironolactone inhibits the growth of cancer stem cells by impairing DNA damage response, Oncogene, 38, 3103-3118, https://doi.org/10.1038/s41388-018-0654-9.
Shen, Y. A., Wang, C. Y., Chuang, H. Y., Hwang, J. J., Chi, W. H., Shu, C. H., Ho, C. Y., Li, W. Y., and Chen, Y. J. (2016) CD44 and CD24 coordinate the reprogramming of nasopharyngeal carcinoma cells towards a cancer stem cell phenotype through STAT3 activation, Oncotarget, 7, 58351-58366, https://doi.org/10.18632/oncotarget.11113.
Ortiz-Montero, P., Liu-Bordes, W. Y., Londono-Vallejo, A., and Vernot, J. P. (2018) CD24 expression and stem-associated features define tumor cell heterogeneity and tumorigenic capacities in a model of carcinogenesis, Cancer Manag. Res., 10, 5767-5784, https://doi.org/10.2147/CMAR.S176654.
Navas, T., Kinders, R. J., Lawrence, S. M., Ferry-Galow, K. V., Borgel, S., Hollingshead, M. G., Srivastava, A. K., Alcoser, S. Y., Makhlouf, H. R., Chuaqui, R., Wilsker, D. F., Konate, M. M., Miller, S. B., Voth, A. R., Chen, L., Vilimas, T., Subramanian, J., Rubinstein, L., Kummar, S., Chen, A. P., et al. (2020) Clinical evolution of epithelial-mesenchymal transition in human carcinomas, Cancer Res., 80, 304-318, https://doi.org/10.1158/0008-5472.CAN-18-3539.
Pacifico, F., Mellone, S., D'Incalci, M., Stornaiuolo, M., Leonardi, A., and Crescenzi, E. (2022) Trabectedin suppresses escape from therapy-induced senescence in tumor cells by interfering with glutamine metabolism, Biochem. Pharmacol., 202, 115159, https://doi.org/10.1016/j.bcp.2022.115159.
Wang, K., Cao, F., Fang, W., Hu, Y., Chen, Y., Ding, H., and Yu, G. (2013) Activation of SNAT1/SLC38A1 in human breast cancer: correlation with p-Akt overexpression, BMC Cancer, 13, 343, https://doi.org/10.1186/1471-2407-13-343.
Wang, M., Liu, Y., Fang, W., Liu, K., Jiao, X., Wang, Z., Wang, J., and Zang, Y. S. (2017) Increased SNAT1 is a marker of human osteosarcoma and potential therapeutic target, Oncotarget, 8, 78930-78939, https://doi.org/10.18632/oncotarget.20693.
Bohme-Schafer, I., Lorentz, S., and Bosserhoff, A. K. (2022) Role of amino acid transporter SNAT1/SLC38A1 in human melanoma, Cancers (Basel), 14, 2151, https://doi.org/10.3390/cancers14092151.
Shishkin, S. S., Eremina, L. S., Kovalev, L. I., and Kovaleva, M. A. (2013) AGR2, ERp57/GRP58, and some other human protein disulfide isomerases, Biochemistry (Moscow), 78, 1415-1430, https://doi.org/10.1134/S000629791313004X.
Maarouf, A., Boissard, A., Henry, C., Leman, G., Coqueret, O., Guette, C., and Lelievre, E. (2022) Anterior gradient protein 2 is a marker of tumor aggressiveness in breast cancer and favors chemotherapyinduced senescence escape, Int. J. Oncol., 60, 5, https://doi.org/10.3892/ijo.2021.5295.
Hrstka, R., Brychtova, V., Fabian, P., Vojtesek, B., and Svoboda, M. (2013) AGR2 predicts tamoxifen resistance in postmenopausal breast cancer patients, Dis. Markers, 35, 207-212, https://doi.org/10.1155/2013/761537.
Li, Z., Zhu, Q., Hu, L., Chen, H., Wu, Z., and Li, D. (2015) Anterior gradient 2 is a binding stabilizer of hypoxia inducible factor-1alpha that enhances CoCl2-induced doxorubicin resistance in breast cancer cells, Cancer Sci., 106, 1041-1049, https://doi.org/10.1111/cas.12714.
Bolesta, E., Pfannenstiel, L. W., Demelash, A., Lesniewski, M. L., Tobin, M., Schlanger, S. E., Nallar, S. C., Papadimitriou, J. C., Kalvakolanu, D. V., and Gastman, B. R. (2012) Inhibition of Mcl-1 promotes senescence in cancer cells: implications for preventing tumor growth and chemotherapy resistance, Mol. Cell Biol., 32, 1879-1892, https://doi.org/10.1128/MCB.06214-11.
Crescenzi, E., Palumbo, G., and Brady, H. J. (2003) Bcl-2 activates a programme of premature senescence in human carcinoma cells, Biochem. J., 375, 263-274, https://doi.org/10.1042/BJ20030868.
Shor, B., Wu, J., Shakey, Q., Toral-Barza, L., Shi, C., Follettie, M., and Yu, K. (2010) Requirement of the mTOR kinase for the regulation of Maf1 phosphorylation and control of RNA polymerase III-dependent transcription in cancer cells, J. Biol. Chem., 285, 15380-15392, https://doi.org/10.1074/jbc.M109.071639.
Kitada, K., Pu, F., and Toi, M. (2019) Occurrence of senescence-escaping cells in doxorubicin-induced senescence is enhanced by PD0332991, a cyclin-dependent kinase 4/6 inhibitor, in colon cancer HCT116 cells, Oncol. Lett., 17, 1153-1159, https://doi.org/10.3892/ol.2018.9657.
Wang, Q., Wu, P. C., Roberson, R. S., Luk, B. V., Ivanova, I., Chu, E., and Wu, D. Y. (2011) Survivin and escaping in therapy-induced cellular senescence, Int. J. Cancer, 128, 1546-1558, https://doi.org/10.1002/ijc.25482.
Han, T. L., Sha, H., Ji, J., Li, Y. T., Wu, D. S., Lin, H., Hu, B., and Jiang, Z. X. (2021) Depletion of Survivin suppresses docetaxel-induced apoptosis in HeLa cells by facilitating mitotic slippage, Sci. Rep., 11, 2283, https://doi.org/10.1038/s41598-021-81563-3.
Zaffaroni, N., Pennati, M., and Daidone, M. G. (2005) Survivin as a target for new anticancer interventions, J. Cell Mol. Med., 9, 360-372, https://doi.org/10.1111/j.1582-4934.2005.tb00361.x.
Le Duff, M., Gouju, J., Jonchere, B., Guillon, J., Toutain, B., Boissard, A., Henry, C., Guette, C., Lelievre, E., and Coqueret, O. (2018) Regulation of senescence escape by the cdk4-EZH2-AP2M1 pathway in response to chemotherapy, Cell Death Dis., 9, 199, https://doi.org/10.1038/s41419-017-0209-y.
Iannetti, A., Ledoux, A. C., Tudhope, S. J., Sellier, H., Zhao, B., Mowla, S., Moore, A., Hummerich, H., Gewurz, B. E., Cockell, S. J., Jat, P. S., Willmore, E., and Perkins, N. D. (2014) Regulation of p53 and Rb links the alternative NF-kappaB pathway to EZH2 expression and cell senescence, PLoS Genet., 10, e1004642, https://doi.org/10.1371/journal.pgen.1004642.
Erokhin, M., Chetverina, O., Gyorffy, B., Tatarskiy, V. V., Mogila, V., Shtil, A. A., Roninson, I. B., Moreaux, J., Georgiev, P., Cavalli, G., and Chetverina, D. (2021) Clinical correlations of polycomb repressive complex 2 in different tumor types, Cancers (Basel), 13, 3155, https://doi.org/10.3390/cancers13133155.
Chien, Y., Scuoppo, C., Wang, X., Fang, X., Balgley, B., Bolden, J. E., Premsrirut, P., Luo, W., Chicas, A., Lee, C. S., Kogan, S. C., and Lowe, S. W. (2011) Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity, Genes Dev., 25, 2125-2136, https://doi.org/10.1101/gad.17276711.
Salunkhe, S., Mishra, S. V., Nair, J., Shah, S., Gardi, N., Thorat, R., Sarkar, D., Rajendra, J., Kaur, E., and Dutt, S. (2021) Nuclear localization of p65 reverses therapy-induced senescence, J. Cell Sci., 134, jcs253203, https://doi.org/10.1242/jcs.253203.
Wang, C., Long, Q., Fu, Q., Xu, Q., Fu, D., Li, Y., Gao, L., Guo, J., Zhang, X., Lam, E. W., Campisi, J., and Sun, Y. (2022) Targeting epiregulin in the treatment-damaged tumor microenvironment restrains therapeutic resistance, Oncogene, 41, 4941-4959, https://doi.org/10.1038/s41388-022-02476-7.
Czabotar, P. E., Lessene, G., Strasser, A., and Adams, J. M. (2014) Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy, Nat. Rev. Mol. Cell Biol., 15, 49-63, https://doi.org/10.1038/nrm3722.
Tse, C., Shoemaker, A. R., Adickes, J., Anderson, M. G., Chen, J., Jin, S., Johnson, E. F., Marsh, K. C., Mitten, M. J., Nimmer, P., Roberts, L., Tahir, S. K., Xiao, Y., Yang, X., Zhang, H., Fesik, S., Rosenberg, S. H., and Elmore, S. W. (2008) ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor, Cancer Res., 68, 3421-3428, https://doi.org/10.1158/0008-5472.CAN-07-5836.
Laberge, R. M., Sun, Y., Orjalo, A. V., Patil, C. K., Freund, A., Zhou, L., Curran, S. C., Davalos, A. R., Wilson-Edell, K. A., Liu, S., Limbad, C., Demaria, M., Li, P., Hubbard, G. B., Ikeno, Y., Javors, M., Desprez, P. Y., Benz, C. C., Kapahi, P., Nelson, P. S., et al. (2015) MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation, Nat. Cell Biol., 17, 1049-1061, https://doi.org/10.1038/ncb3195.
Ovadya, Y., and Krizhanovsky, V. (2018) Strategies targeting cellular senescence, J. Clin. Invest., 128, 1247-1254, https://doi.org/10.1172/JCI95149.
Wyld, L., Bellantuono, I., Tchkonia, T., Morgan, J., Turner, O., Foss, F., George, J., Danson, S., and Kirkland, J. L. (2020) Senescence and cancer: a review of clinical implications of senescence and senotherapies, Cancers (Basel), 12, 2134, https://doi.org/10.3390/cancers12082134.
Zhu, Y., Tchkonia, T., Fuhrmann-Stroissnigg, H., Dai, H. M., Ling, Y. Y., Stout, M. B., Pirtskhalava, T., Giorgadze, N., Johnson, K. O., Giles, C. B., Wren, J. D., Niedernhofer, L. J., Robbins, P. D., and Kirkland, J. L. (2016) Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors, Aging Cell, 15, 428-435, https://doi.org/10.1111/acel.12445.
Grezella, C., Fernandez-Rebollo, E., Franzen, J., Ventura Ferreira, M. S., Beier, F., and Wagner, W. (2018) Effects of senolytic drugs on human mesenchymal stromal cells, Stem Cell Res. Ther., 9, 108, https://doi.org/10.1186/s13287-018-0857-6.
Zhu, Y., Tchkonia, T., Pirtskhalava, T., Gower, A. C., Ding, H., Giorgadze, N., Palmer, A. K., Ikeno, Y., Hubbard, G. B., Lenburg, M., O’Hara, S. P., LaRusso, N. F., Miller, J. D., Roos, C. M., Verzosa, G. C., LeBrasseur, N. K., Wren, J. D., Farr, J. N., Khosla, S., Stout, M. B., et al. (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs, Aging Cell, 14, 644-658, https://doi.org/10.1111/acel.12344.
Sharma, A. K., Roberts, R. L., Benson, R. D., Jr., Pierce, J. L., Yu, K., Hamrick, M. W., and McGee-Lawrence, M. E. (2020) The senolytic drug navitoclax (ABT-263) causes trabecular bone loss and impaired osteoprogenitor function in aged mice, Front. Cell Dev. Biol., 8, 354, https://doi.org/10.3389/fcell.2020.00354.
Zhu, Y., Doornebal, E. J., Pirtskhalava, T., Giorgadze, N., Wentworth, M., Fuhrmann-Stroissnigg, H., Niedernhofer, L. J., Robbins, P. D., Tchkonia, T., and Kirkland, J. L. (2017) New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463, Aging (Albany NY), 9, 955-963, https://doi.org/10.18632/aging.101202.
Yousefzadeh, M. J., Zhu, Y., McGowan, S. J., Angelini, L., Fuhrmann-Stroissnigg, H., Xu, M., Ling, Y. Y., Melos, K. I., Pirtskhalava, T., Inman, C. L., McGuckian, C., Wade, E. A., Kato, J. I., Grassi, D., Wentworth, M., Burd, C. E., Arriaga, E. A., Ladiges, W. L., Tchkonia, T., Kirkland, J. L., et al. (2018) Fisetin is a senotherapeutic that extends health and lifespan, EBioMedicine, 36, 18-28, https://doi.org/10.1016/j.ebiom.2018.09.015.
Li, J., Gong, X., Jiang, R., Lin, D., Zhou, T., Zhang, A., Li, H., Zhang, X., Wan, J., Kuang, G., and Li, H. (2018) Fisetin inhibited growth and metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via PTEN/Akt/GSK3beta signal pathway, Front. Pharmacol., 9, 772, https://doi.org/10.3389/fphar.2018.00772.
Youns, M., and Abdel Halim Hegazy, W. (2017) The natural flavonoid fisetin inhibits cellular proliferation of hepatic, colorectal, and pancreatic cancer cells through modulation of multiple signaling pathways, PLoS One, 12, e0169335, https://doi.org/10.1371/journal.pone.0169335.
Khan, N., Jajeh, F., Eberhardt, E. L., Miller, D. D., Albrecht, D. M., Van Doorn, R., Hruby, M. D., Maresh, M. E., Clipson, L., Mukhtar, H., and Halberg, R. B. (2019) Fisetin and 5-fluorouracil: Effective combination for PIK3CA-mutant colorectal cancer, Int. J. Cancer, 145, 3022-3032, https://doi.org/10.1002/ijc.32367.
Zhuo, W., Zhang, L., Zhu, Y., Zhu, B., and Chen, Z. (2015) Fisetin, a dietary bioflavonoid, reverses acquired Cisplatin-resistance of lung adenocarcinoma cells through MAPK/Survivin/Caspase pathway, Am. J. Transl. Res., 7, 2045-2052.
Porter, D. C., Farmaki, E., Altilia, S., Schools, G. P., West, D. K., Chen, M., Chang, B. D., Puzyrev, A. T., Lim, C. U., Rokow-Kittell, R., Friedhoff, L. T., Papavassiliou, A. G., Kalurupalle, S., Hurteau, G., Shi, J., Baran, P. S., Gyorffy, B., Wentland, M. P., Broude, E. V., Kiaris, H., et al. (2012) Cyclin-dependent kinase 8 mediates chemotherapy-induced tumor-promoting paracrine activities, Proc. Natl. Acad. Sci. USA, 109, 13799-13804, https://doi.org/10.1073/pnas.1206906109.
Sharko, A. C., Lim, C. U., McDermott, M. S. J., Hennes, C., Philavong, K. P., Aiken, T., Tatarskiy, V. V., Roninson, I. B., and Broude, E. V. (2021) The inhibition of CDK8/19 mediator kinases prevents the development of resistance to EGFR-targeting drugs, Cells, 10, 144, https://doi.org/10.3390/cells10010144.
Sanchez-Diaz, L., Espinosa-Sanchez, A., Blanco, J. R., and Carnero, A. (2022) Senotherapeutics in cancer and HIV, Cells, 11, 1222, https://doi.org/10.3390/cells11071222.
Serra, F., Lapidari, P., Quaquarini, E., Tagliaferri, B., Sottotetti, F., and Palumbo, R. (2019) Palbociclib in metastatic breast cancer: current evidence and real-life data, Drugs Context, 8, 212579, https://doi.org/10.7573/dic.212579.
Galardi, F., De Luca, F., Biagioni, C., Migliaccio, I., Curigliano, G., Minisini, A. M., Bonechi, M., Moretti, E., Risi, E., McCartney, A., Benelli, M., Romagnoli, D., Cappadona, S., Gabellini, S., Guarducci, C., Conti, V., Biganzoli, L., Di Leo, A., and Malorni, L. (2021) Circulating tumor cells and palbociclib treatment in patients with ER-positive, HER2-negative advanced breast cancer: results from a translational sub-study of the TREnd trial, Breast Cancer Res., 23, 38, https://doi.org/10.1186/s13058-021-01415-w.
Jost, T., Heinzerling, L., Fietkau, R., Hecht, M., and Distel, L. V. (2021) Palbociclib induces senescence in melanoma and breast cancer cells and leads to additive growth arrest in combination with irradiation, Front. Oncol., 11, 740002, https://doi.org/10.3389/fonc.2021.740002.
Bi, H., Shang, J., Zou, X., Xu, J., and Han, Y. (2021) Palbociclib induces cell senescence and apoptosis of gastric cancer cells by inhibiting the Notch pathway, Oncol. Lett., 22, 603, https://doi.org/10.3892/ol.2021.12864.
Rubinsztein, D. C., Codogno, P., and Levine, B. (2012) Autophagy modulation as a potential therapeutic target for diverse diseases, Nat. Rev. Drug Discov., 11, 709-730, https://doi.org/10.1038/nrd3802.
Lee, H. O., Mustafa, A., Hudes, G. R., and Kruger, W. D. (2015) Hydroxychloroquine destabilizes phospho-S6 in human renal carcinoma cells, PLoS One, 10, e0131464, https://doi.org/10.1371/journal.pone.0131464.
Harnicek, D., Kampmann, E., Lauber, K., Hennel, R., Cardoso Martins, A. S., Guo, Y., Belka, C., Mortl, S., Gallmeier, E., Kanaar, R., Mansmann, U., Hucl, T., Lindner, L. H., Hiddemann, W., and Issels, R. D. (2016) Hyperthermia adds to trabectedin effectiveness and thermal enhancement is associated with BRCA2 degradation and impairment of DNA homologous recombination repair, Int. J. Cancer, 139, 467-479, https://doi.org/10.1002/ijc.30070.
Camorani, S., Cerchia, L., Fedele, M., Erba, E., D’Incalci, M., and Crescenzi, E. (2018) Trabectedin modulates the senescence-associated secretory phenotype and promotes cell death in senescent tumor cells by targeting NF-kappaB, Oncotarget, 9, 19929-19944, https://doi.org/10.18632/oncotarget.24961.
Funding
This work was financially supported by the Russian Science Foundation (grant no. 22-24-00212).
Author information
Authors and Affiliations
Contributions
Zamkova, M. A. – writing and editing the text; Persiyantseva, N. A., Tatarskiy, V. V. – editing the text; Shtil, A. A. – conceptualization, writing, and final editing.
Corresponding author
Ethics declarations
The authors declare no conflict of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Zamkova, M.A., Persiyantseva, N.A., Tatarskiy, V.V. et al. Therapy-Induced Tumor Cell Senescence: Mechanisms and Circumvention. Biochemistry Moscow 88, 86–104 (2023). https://doi.org/10.1134/S000629792301008X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000629792301008X