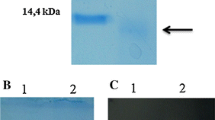
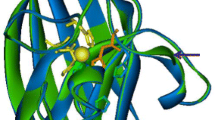
Abstract
A chimeric gene construct encoding human peroxiredoxin 6 and Mn-superoxide dismutase from Escherichia coli was developed. Conditions for expression of the fusion protein in E. coli cell were optimized. Fusing of the enzymes into a single polypeptide chain with peroxiredoxin 6 at the N-terminus (PSH) did not affect their activities. On the contrary, the chimeric protein with reverse order of enzymes (SPH) was not obtained in a water-soluble active form. The active chimeric protein (PSH) exhibiting both peroxidase and superoxide dismutase activities was prepared and its physicochemical properties were characterized.
Similar content being viewed by others
References
Halliwell, B., and Gutteridge, J. M. (2007) Free Radicals in Biology and Medicine, 4th Edn., Oxford University Press.
Menshchikova, E. B., Lankin, V. Z., Zenkov, N. K., Bondar, I. A., Krugovykh, N. F., and Trufakin, V. A. (2006) Oxidative Stress. Prooxidants and Antioxidants [in Russian], Slovo, Moscow.
Bruskov, V. I., Karp, O. E., Garmash, S. A., Shtarkman, I. N., Chernikov, A. V., and Gudkov, S. V. (2012) Prolongation of oxidative stress by long-lived reactive protein species induced by X-ray radiation and their genotoxic action, Free Radic. Res., 46, 1280–1290.
Maksimenko, A. V. (2005) Experimental antioxidant biotherapy for protection of the vascular wall by modified forms of superoxide dismutase and catalase, Curr. Pharm. Des., 11, 2007–2016.
Maksimenko, A. V., Golubykh, V. L., and Tischenko, E. G. (2004) The combination of modified antioxidant enzymes for anti-thrombotic protection of the vascular wall: the significance of covalent connection of superoxide dismutase and catalase activities, J. Pharm. Pharmacol., 56, 1463–1468.
Stenlund, P., and Tibell, L. A. (1999) Chimeras of human extracellular and intracellular superoxide dismutases. Analysis of structure and function of the individual domains, Protein Eng., 12, 319–325.
Isarankura-Na-Ayudhya, C., Yainoy, S., Tantimongcolwat, T., Bulow, L., and Prachayasittikul, V. (2010) Engineering of a novel chimera of superoxide dismutase and Vitreoscilla hemoglobin for rapid detoxification of reactive oxygen species, J. Biosci. Bioeng., 110, 633–637.
Seetharaman, S. V., Taylor, A. B., Holloway, S., and Hart, P. J. (2010) Structures of mouse SOD1 and human/mouse SOD1 chimeras, Arch. Biochem. Biophys., 503, 183–190.
Elchuri, S., Oberley, T. D., Qi, W., Eisenstein, R. S., Jackson Roberts, L., Van Remmen, H., Epstein, C. J., and Huang, T. T. (2005) Cu,Zn-SOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life, Oncogene, 24, 367–380.
Li, Y., Huang, T. T., Carlson, E. J., Melov, S., Ursell, P. C., Olson, J. L., Noble, L. J., Yoshimura, M. P., Berger, C., Chan, P. H., Wallace, D. C., and Epstein, C. J. (1995) Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase, Nat. Genet., 11, 376–381.
Sentman, M. L., Granstrom, M., Jakobson, H., Reaume, A., Basu, S., and Marklund, S. L. (2006) Phenotypes of mice lacking extracellular superoxide dismutase and copperand zinc-containing superoxide dismutase, J. Biol. Chem., 281, 6904–6909.
Michelson, A. M., Puget, K., and Jadot, G. (1986) Antiinflammatory activity of superoxide dismutases: comparison of enzymes from different sources in different models in rats: mechanism of action, Free Radic. Res. Commun., 2, 43–56.
Keele, B. B., Jr., Mc Cord, J. M., and Fridovich, I. (1970) Superoxide dismutase from Escherichia coli B. A new manganese-containing enzyme, J. Biol. Chem., 245, 6176–6181.
Beyer, W. F., Jr., and Fridovich, I. (1987) Effect of hydrogen peroxide on the iron-containing superoxide dismutase of Escherichia coli, Biochemistry, 26, 1251–1257.
Sheng, Y., Abreu, I. A., Cabelli, D. E., Maroney, M. J., Miller, A. F., Teixeira, M., and Valentine, J. S. (2014) Superoxide dismutases and superoxide reductases, Chem. Rev., 114, 3854–3918.
Wood, Z. A., Poole, L. B., and Karplus, P. A. (2003) Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling, Science, 300, 650–653.
Sharapov, M. G., Ravin, V. K., and Novoselov, V. I. (2014) Peroxiredoxins as multifunctional enzymes, Mol. Biol. (Moscow), 48, 600–628.
Chen, J. W., Dodia, C., Feinstein, S. I., Jain, M. K., and Fisher, A. B. (2000) 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities, J. Biol. Chem., 275, 28421–28427.
Manevich, Y., Shuvaeva, T., Dodia, C., Kazi, A., Feinstein, S. I., and Fisher, A. B. (2009) Binding of peroxiredoxin 6 to substrate determines differential phospholipid hydroperoxide peroxidase and phospholipase A2 activities, Arch. Biochem. Biophys., 485, 139–149.
Nagy, N., Malik, G., Fisher, A. B., and Dipak, K. D. (2006) Targeted disruption of peroxiredoxin 6 gene renders the heart vulnerable to ischemia-reperfusion injury, Am. J. Physiol. Heart Circ. Physiol., 291, 2636–2640.
Kumin, A., Huber, C., Rulice, T., Wolf, E., and Werner, S. (2006) Peroxiredoxin 6 is a potent cytoprotective enzyme in the epidermis, Am. J. Pathol., 169, 1194–1205.
Novoselov, V. I., Ravin, V. K., Sharapov, M. G., Sofin, A. D., Kukushkin, N. I., and Fesenko, E. E. (2011) Modified peroxiredoxins as prototypes of drugs with powerful antioxidant action, Biophysics (Moscow), 56, 873–880.
Volkova, A. G., Sharapov, M. G., Ravin, V. K., Gordeeva, A. E., Karaduleva, E. A., Mubarakshina, E. K., Temnov, A. A., Fesenko, E. E., and Novoselov, V. I. (2014) Effect of various enzymes antioxidants on tracheal epithelial regeneration following chemical burn, Pulmonologiya, 12, 84–90.
Novoselov, V. I., Amelina, S. E., Kravchenko, I. N., Novoselov, S. V., Yanin, V. A., Sadovnikov, V. B., and Fesenko, E. E. (2000) The role of peroxiredoxin in the antioxidant system of respiratory organs, Dokl. Biophys., 373-375, 64–66.
Chuchalin, A. G., Novoselov, V. I., Shifrina, O. N., Soodaeva, S. K., Yanin, V. A., and Barishnikova, L. M. (2003) Peroxiredoxin VI in human respiratory system, Respir. Med., 97, 147–151.
Manevich, Y., and Fisher, A. B. (2005) Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism, Free Radic. Biol. Med., 38, 1422–1432.
Sambrook, J., and Russell, D. W. (2001) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, Cold Spring Harbor Laboratory Press, N. Y.
Kang, S. W., Baines, I. C., and Rhee, S. G. (1998) Characterization of a mammalian peroxiredoxin that contains one conserved cysteine, J. Biol. Chem., 273, 6303–6311.
Sharapov, M. G., Novoselov, V. I., and Ravin, V. K. (2009) The cloning, expression, and comparative analysis of peroxiredoxin 6 from various sources, Mol. Biol. (Moscow), 43, 505–511.
Yourno, J., Kohno, T., and Roth, J. R. (1970) Enzyme evolution: generation of a bifunctional enzyme by fusion of adjacent genes, Nature, 228, 820–824.
Bulow, L. (1990) Preparation of artificial bifunctional enzymes by gene fusion, Biochem. Soc. Symp., 57, 123–133.
Zhang, W., Fisher, J. F., and Mobashery, S. (2009) The bifunctional enzymes of antibiotic resistance, Curr. Opin. Microbiol., 12, 505–511.
Luo, G., Liu, W., Sun, Q., Ding, L., Zhu, Z., Yan, G., and Yang, T. (1996) Preparation of bifunctional enzyme with both superoxide dismutase and glutathione peroxidase activities by using chemical mutation, Ann. N. Y. Acad. Sci., 50, 799.
Yan, F., Yang, W. K., Li, X. Y., Lin, T. T., Lun, Y. N., Lin, F., Lu, S. W., Yan, G. L., Liu, J. Q., Shen, J. C., Mu, Y., and Luo, G. M. (2008) A trifunctional enzyme with glutathione S-transferase, glutathione peroxidase and superoxide dismutase activity, Biochim. Biophys. Acta, 1780, 869–872.
Fisher, A. B. (2011) Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities, Antioxid. Redox Signal., 15, 831–844.
Choi, H. J., Kang, S. W., Yang, C. H., Rhee, S. G., and Ryu, S. E. (1998) Crystal structure of a novel human peroxidase enzyme at 2.0 Å resolution, Nat. Struct. Biol., 5, 400–406.
Smeets, A., Loumaye, E., Clippe, A., Rees, J. F., Knoops, B., and Declercq, J. P. (2008) The crystal structure of the C45S mutant of annelid Arenicola marina peroxiredoxin 6 supports its assignment to the mechanistically typical 2-Cys subfamily without any formation of toroid-shaped decamers, Protein Sci., 17, 700–710.
Nekrasov, A. N., Radchenko, V. V., Shuvaeva, T. M., Novoselov, V. I., Fesenko, E. E., and Lipkin, V. M. (2007) The novel approach to the protein design: active truncated forms of human 1-CYS peroxiredoxin, J. Biomol. Struct. Dyn., 24, 455–462.
Radchenko, V. V., Nekrasov, A. N., Shuvaeva, T. M., and Lipkin, V. M. (2005) Information Structure Analysis of Protein Sequences. Design and Purification of Recombinant Truncated Forms of Human 1-CYS Peroxiredoxin (PrxVI), Moscow Conf. on Computational Molecular Biology, Moscow, pp. 311–313.
Sheng, Y., Abreu, I. A., Cabelli, D. E., Maroney, M. J., Miller, A. F., Teixeira, M., and Valentine, J. S. (2014) Superoxide dismutases and superoxide reductases, Chem. Rev., 114, 3854–3918.
Palutina, O. A., Sharapov, M. G., Temnov, A. A., and Novoselov, V. I (2016) Nephroprotective effect of exogenous antioxidant enzymes during ischemia/reperfusioninduced damage of renal tissue, Bull. Exp. Biol. Med., 160, 322–326.
Karaduleva, E. V., Mubarakshina, E. K., Sharapov, M. G., Volkova, A. E., Pimenov, O. Yu., Ravin, V. K., Kokoz, Yu. M., and Novoselov, V. I. (2016) Cardioprotective effect of modified peroxiredoxins during retrograde perfusion of isolated rat heart under oxidative stress conditions, Bull. Exp. Biol. Med., 160, 584–588.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M. G. Sharapov, V. I. Novoselov, V. K. Ravin, 2016, published in Biokhimiya, 2016, Vol. 81, No. 4, pp. 571-579.
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM15-400, February 14, 2016.
Rights and permissions
About this article
Cite this article
Sharapov, M.G., Novoselov, V.I. & Ravin, V.K. Construction of a fusion enzyme exhibiting superoxide dismutase and peroxidase activity. Biochemistry Moscow 81, 420–427 (2016). https://doi.org/10.1134/S0006297916040131
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297916040131