Abstract
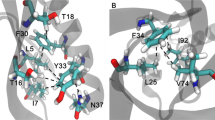
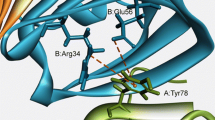
Cation-π interactions are known to be important contributors to protein stability and ligand-protein interactions. In this study, we have analyzed the influence of cation-π interactions in single chain immunoglobulin proteins. We observed 87 cation-π interactions in a data set of 33 proteins. These interactions are mainly formed by long-range contacts, and there is preference of Arg over Lys in these interactions. Arg-Tyr interactions are predominant among the various pairs analyzed. Despite the scarcity of interactions involving Trp, the average energy for Trp-cation interactions is quite high. This information suggests that the cation-π interactions involving Trp might be of high relevance to the proteins. Secondary structure analysis reveals that cation-π interactions are formed preferably between residues in which at least one is in β-strand. Proteins having β-strand regions have the highest number of cation-π interaction-forming residues.
Similar content being viewed by others
Abbreviations
- ASA:
-
accessible surface area
- LRO:
-
long-range order
- SC:
-
stabilizing centers
References
Gromiha, M. M., Thomas, S., and Santhosh, C. (2002) Prep. Biochem. Biotech., 32, 355–362.
Chakravarty, S., and Varadarajan, R. (2000) Biochemistry, 41, 8152–8161.
Shi, Z., Olson, C. A., and Kallenbach, N. R. (2002) J. Am. Chem. Soc., 124, 3284–3291.
Burghardt, T. P., Juranic, N., Macura, S., and Ajtai, K. (2002) Biopolymers, 63, 261–272.
Mulhern, T. D., Lopez, A. F., D’Andrea, R. J., Gaunt, C., Vandeleur, L., Vadas, M. A., Booker, G. W., and Bagley, C. J. (2000) J. Mol. Biol., 297, 989–1001.
Gromiha, M. M. (2003) Biophys. Chem., 103, 251–258.
Harpaz, Y., and Chothia, C. (1994) J. Mol. Biol., 238, 528–539.
Barclay, A. (2003) Semin. Immunol., 15, 215–223.
Hunter, C. A., and Sanders, J. K. M. (1990) J. Am. Chem. Soc., 112, 5525–5534.
Dill, K. A. (1990) Biochemistry, 29, 7133–7155.
Rose, G. D., and Wolfenden, R. (1993) Biophys. Biomol. Struct., 22, 381–415.
Ponnuswamy, P. K., and Gromiha, M. M. (1994) J. Theor. Biol., 166, 63–74.
Pace, C. N. (1995) Meth. Enzymol., 259, 538–554.
Dosztanyi, Z., Fiser, A., and Simon, I. (1997) J. Mol. Biol., 272, 597–612.
Gromiha, M. M., and Selvaraj, S. (2001) J. Mol. Biol., 310, 27–32.
Poupon, A., and Mornon, J. P. (1999) FEBS Lett., 452, 283–289.
Zacharias, N., and Dougherty, D. A. (2002) Trends Pharmacol. Sci., 23, 281–287.
Zhong, W., Gallivan, J. P., Zhang, Y., Li, L., Lester, H. A., and Dougherty, D. A. (1998) Proc. Natl. Acad. Sci. USA, 95, 12088–12093.
Scrutton, N. S., and Raine, A. R. C. (2000) Biochem. J., 319, 1–8.
Liu, R., Pidikiti, R., Petersen, C. E., Bhagavan, N. V., and Eckenhoff, R. G. (2002) J. Biol. Chem., 277, 36373–36379.
Ma, J. C., and Dougherty, D. A. (1997) Chem. Rev., 97, 1303–1324.
Gromiha, M. M., Santhosh, C., and Suwa, M. (2004) Polymer, 45, 633–639.
Gromiha, M. M. (2005) Polymer, 46, 983–990.
Anand, A., Sudha, A., Lazar Mathew, and Sethumadhavan, R. (2006) Cytokine, 35, 263–269.
Anand, A., Sudha, A., Lazar Mathew, and Sethumadhavan, R. (2007) Int. J. Biol. Macromol., 40, 479–483.
Berman, H. M., Westbrook, J., Feng, Z., et al. (2000) Nucleic Acids Res., 28, 235–242.
Murzin, A. G., and Brenner, S. E., Hubbard, T., and Chothia, C. (1995) J. Mol. Biol., 247, 536–540.
Gallivan, J. P., and Dougherty, D. A. (1999) Proc. Natl. Acad. Sci. USA, 96, 9459–9464.
Jorgensen, W. L., Maxwell, D. S., and Rives, J. T. (1996) J. Am. Chem. Soc., 118, 11225–11236.
Gromiha, M. M., and Selvaraj, S. (2004) Biophys. Mol. Biol., 86, 235–277.
Kabsch, W., and Sander, C. (1983) Biopolymers, 22, 2577–2637.
Heringa, J., and Argos, P. (1989) Proteins, 37, 30–43.
Dosztanyi, Z. S., Magyar, C. S., Tusnady, E., and Simon, I. (2003) Bioinformatics, 19, 899–900.
Gromiha, M. M., Pujadas, G., Magyar, C., Selvaraj, S., and Simon, I. (2004) Proteins, 55, 316–329.
Glaser, F., Pupko, T., Paz, I., Bell, R. E., Bechor, D., Martz, E., and Ben-Tal, N. (2003) Bioinformatics, 19, 163–164.
Boeckman, B., Bairoch, A., Apweiler, R., Blatter, M. C., Estreicher, A., Gasteiger, E., Martin, M. J., Michoud, K., O’Donovan, C., Phan, I., Pilbout, S., and Schneider, M. (2003) Nucleic Acids Res., 31, 365–370.
Gromiha, M. M., Oobatake, M., Kono, H., Uedaira, H., and Sarai, A. (1999) Protein Eng., 12, 549–555.
Gilis, D., and Rooman, M. (1997) J. Mol. Biol., 272, 276–290.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tayubi, I.A., Sethumadhavan, R. Nature of cation-π interactions and their role in structural stability of immunoglobulin proteins. Biochemistry Moscow 75, 912–918 (2010). https://doi.org/10.1134/S000629791007014X
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000629791007014X