Abstract
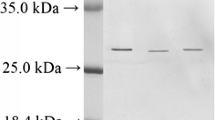
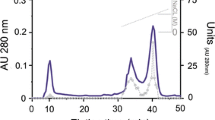
Two new serine proteinase inhibitors (RmIn I and RmIn II) from the tropical sea anemone Radianthus macrodactylus have been isolated and characterized. The purification procedure includes polychrome-1 hydrophobic chromatography, Superdex™ Peptide 10/30 FPLC, and Nucleosil C18 reverse-phase HPLC. The molecular masses of RmIn I, RmIn II, and the complexes RmIn II/trypsin and RmIn I,II/α-chymotrypsin have been determined. The K i values of RmIn I and RmIn II for trypsin and α-chymotrypsin have been determined. The polypeptides RmIn I and RmIn II are shown to be nontoxic and to exhibit antihistamine activity. The N-terminal amino acid sequences of RmIn I (GICSEPIVVGPCKAG-) and RmIn II (GSTCLEPKVVGPCKA-) have been determined. A high homology of the amino acid sequences is demonstrated for the proteinase inhibitors produced by such evolutionarily distant species as coelenterates, reptiles, and mammals.
Similar content being viewed by others
Abbreviations
- BAPNA:
-
N-benzoyl-D,L-arginine p-nitroanilide
- BSA:
-
bovine serum albumin
- BTEE:
-
N-benzoyl-L-tyrosine ethyl ester
- HPLC:
-
high performance liquid chromatography
- TFA:
-
trifluoroacetic acid
References
Mosolov, V. V., and Valueva, T. A. (2005) Prikl. Biokhim. Mikrobiol., 41, 261–282.
Rui-Feng Qi, Zhan-Wu Song, and Cheng-Wu Chi (2005) Acta Biochim. Biophys. Sinica, 37, 283–292.
Bode, W., and Huber, R. (2002) Biochim. Biophys. Acta, 1477, 241–252.
Krowarsch, D., Cierpicki, T., Jelen, F., and Otlewski, J. (2003) CMLS, 60, 2427–2444.
Janciauskiene, S. (2001) Biochim. Biophys. Acta, 1535, 221–235.
Gettins, P. G. (2002) Chem. Rev., 102, 4751–4804.
Fritz, H., Brey, B., and Beress, L. (1972) Hoppe-Seyler’s Z. Physiol. Chem., 353, 19–30.
Wunderer, G., Beress, L., Machleidt, W., and Fritz, H. (1976) Meth. Enzymol., 45, 881–885.
Zykova, T. A., Vinokurov, L. M., Markova, L. F., Kozlovskaya, E. P., and Elyakov, G. B. (1985) Bioorg. Khim., 11, 293–301.
Shvets, T. V., Monastyrnaya, M. M., Zykova, T. A., and Kozlovskaya, E. P. (2000) Abstr. II Int. Symp. “Chemistry and Chemical Education”, DVGU Press, Vladivostok, p. 233.
Shiomi, K., Ishikawa, M., Yamanaka, H., and Kikuchi, T. (1989) Nippon Suisan Gakkaishi, 55, 1235–1241.
Ishida, M., Minagawa, S., Miyauchi, K., Shimakura, K., Nagashima, Y., and Shiomi, K. (1997) Fish. Sci., 63, 794–798.
Delfin, J., Martinez, I., Antuch, W., Morera, V., Gonzalez, Y., Rodriguez, R., Marquez, M., Saroyan, A., Larionova, N., Diaz, J., Padron, G., and Chavez, M. (1996) Toxicon, 34, 1367–1376.
Diaz, J., Morera, V., Delfin, J., Huerta, V., Lima, G., Rodriguez de la Vega, M., Garcia, B., Padron, G., Assfalg-Machleidt, I., Machleidt, W., and Chavez, M. (1998) Toxicon, 36, 1275–1276.
Minagawa, S., Ishida, M., Shimakura, K., Nagashima, Y., and Shiomi, K. (1997) Comp. Biochem. Physiol. B. Biochem. Mol. Biol., 118, 381–386.
Schweitz, H., Bruhn, T., Guillemare, E., Moinier, D., Lancelin, J.-M., Beves, L., and Lazdunski, M. (1995) J. Biol. Chem., 270, 25121–25126.
Mebs, D., and Gebauer, E. (1980) Toxicon, 18, 97–106.
Mebs, D., Liebrich, M., Reul, A., and Samejima, Y. (1983) Toxicon, 21, 257–264.
Lowry, O. H., Rosebrough, N. J., Farr, A. I., and Randall, R. J. (1951) J. Biol. Chem., 193, 265–275.
Laemmli, U. K. (1970) Nature, 227, 680–682.
Dixon, M., and Webb, E. (1961) in Enzymes (Oparin, A. I., ed.) [Russian translation], Inostrannaya Literatura, Moscow, pp. 30–32.
Gatsura, V. V. (1974) in Methods of the Primary Pharmacological Investigation of Biologically Active Compounds (Kalinkina, M. V., ed.) [in Russian], Meditsina, Moscow, pp. 74–76.
Ritonja, A., Meloun, B., and Gubensek, F. (1983) Biochim. Biophys. Acta, 748, 429–435.
Cechova, D., Jonakova, V., and Sorm, F. (1971) Collect. Czech. Chem. Commun., 36, 3342–3357.
Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994) Nucleic Acid Res., 22, 4673–4680.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I. N. Sokotun, A. P. Il’ina, M. M. Monastyrnaya, E. V. Leychenko, A. A. Es’kov, S. D. Anastuk, E. P. Kozlovskaya, 2007, published in Biokhimiya, 2007, Vol. 72, No. 3, pp. 368–374.
Rights and permissions
About this article
Cite this article
Sokotun, I.N., Il’ina, A.P., Monastyrnaya, M.M. et al. Proteinase inhibitors from the tropical sea anemone Radianthus macrodactylus: Isolation and characteristic. Biochemistry Moscow 72, 301–306 (2007). https://doi.org/10.1134/S0006297907030078
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1134/S0006297907030078