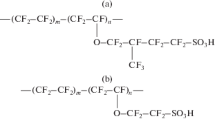Abstract
The free solvent transport number in an MF-4SK perfluorinated membrane in solutions of alkaline metal chlorides and hydrochloric acid is for the first time calculated within the framework of a capillary model based on the data of standard contact porosimetry and membrane conductometry. The reasons for the change in the structural characteristics and specific conductivity upon varying the nature of the counterion are discussed. The portion of through mesopores in MF-4SK homogeneous and MK-40 heterogeneous sulfonated cation-exchange membranes is estimated using the experimental data on the water transport numbers in solutions of electrolytes of different natures.







Similar content being viewed by others
REFERENCES
M. Yaqub and W. Lee, Sci. Total Environ. 681, 551 (2019).
J. Havelka, H. Fárová, T. Jiříček, T. Kotala, and J. Kroupa, Water Sci. Technol. 79, 1580 (2019).
G. J. Doornbusch, M. Tedesco, J. W. Post, Z. Borneman, and K. Nijmeijer, Desalination 464, 105 (2019).
K. V. Protasov, S. A. Shkirskaya, N. P. Berezina, and V. I. Zabolotskii, Russ. J. Electrochem. 46, 1131 (2010).
L. Han, S. Galier, and H. Roux-de Balmann, Desalination 373, 38 (2015).
A. H. Galama, M. Saakes, H. Bruning, H. H. M. Rijnaarts, and J. W. Post, Desalination 342, 61 (2013).
B. Sun, M. Zhang, S. Huang, Z. Cao, L. Lu, and X. Zhang, Sep. Purif. Technol. 281, 119907 (2022).
B. Sun, M. Zhang, S. Huang, J. Wang, X. Zhang, Desalination 498, 114793 (2021).
S. Porada, W. J. van Egmond, J. W. Post, M. Saakes, and H. V. M. Hamelers, J. Membr. Sci. 552, 22 (2018).
J. O. Bockris and K. N. Reddy, Modern Electrochemistry. Ionics (Kluwer Academic Publishers, London, 2002).
G. Xie and T. Okada, Electrochim. Acta 41, 1569 (1996).
R. Sprocati and M. Rolle, Water Res. 213, 118161 (2022).
T. Yamanaka, T. Takeguchi, H. Takahashi, and W. Ueda, J. Electrochem. Soc. 156, B831 (2009).
J. Garrido, V. Compan, M. L. Lopez, and D. G. Miller, J. Phys. Chem. 101, 5740 (1997).
C. Larchet, B. Auclair, and V. Nikonenko, Electrochim. Acta 49, 1711 (2004).
H. M. Park and Y. J. Choi, Int. J. Heat Mass Transfer 52, 4279 (2009).
P. Schaetzel, Q. T. Nguyen, and B. Riffault, J. Membr. Sci. 240, 25 (2004).
A. N. Filippov, Colloid J. 80, 716 (2018).
Y. Xin, Y.-X. Zheng, and Y.-X. Yu, Mol. Phys. 114, 2328 (2016).
P. Meares, J. Polymer Science 20, 507 (1956).
I. V. Falina, V. I. Zabolotsky, O. A. Demina, and N. V. Sheldeshov, J. Membr. Sci. 573, 520 (2019).
Yu. M. Vol’fkovich, Elektrokhimiya 20, 669 (1984).
N. P. Berezina, S. A. Shkirskaya, A. A.-R. Sycheva, and M. V. Krishtopa, Colloid J. 71, 397 (2008).
N. Kononenko, V. Nikonenko, D. Grande, C. Larchet, L. Dammak, M. Fomenko, and Yu. Volfkovich, Adv. Colloid Interface Sci. 246, 196 (2017).
M. I. Bakeev, Hydration and Physicochemical Properties of Electrolyte Solutions (Nauka, Alma-Ata, 1978) [in Russian].
I. T. Goronovskii, Yu. P. Nazarenko, and E. F. Nekryach, Quick Reference Guide to Chemistry (Naukova Dumka, Kiev, 1987) [in Russian].
R. A Robinson and R. H. Stoks, Electrolyte Solutions, 2nd Ed. (Dover Publications, 2002).
Handbook of Electrochemistry, Ed. by A. M. Sukhotin (Khimiya, Leningrad, 1981) [in Russian].
N. P. Berezina, N. A. Kononenko, O. A. Dyomina, and N. P. Gnusin, Adv. Colloid Interface Sci. 139, 3 (2008).
E. Yu. Safronova, V. I. Volkov, A. A. Pavlov, A. V. Chernyak, E. V. Volkov, and A. B. Yaroslavtsev, Russ. J. Inorg. Chem. 56, 156 (2011).
N. H. Jalani and R. Datta, J. Membr. Sci. 264, 167 (2005).
I. A. Stenina, P. Sistat, A. I. Rebrov, G. Pourcelly, and A. B. Yaroslavtsev, Desalination 170, 49 (2004).
G. Pourcelly, A. Oikonomou, and C. Gavach, J. Electroanal. Chem. 287, 43 (1990).
V. I. Volkov, E. V. Volkov, S. V. Timofeev, E. A. Sanginov, A. A. Pavlov, E. Yu. Safronova, I. A. Stenina, and A. B. Yaroslavtsev, Russ. J. Inorg. Chem. 55, 315 (2010).
I. V. Falina, O. A. Demina, and V. I. Zabolotskii, Membr. Membr. Tekhnol. 9, 81 (2019).
A. N. Filippov, E. Yu. Safronova, and A. B. Yaroslavtsev, J. Membr. Sci. 471, 110 (2014).
K. D. Kreuer, S. J. Paddison, E. Spohr, and M. Schuster, Chem. Rev. 104, 4637 (2004).
Funding
This research was financially supported by the Kuban Science Foundation within scientific project no. H-21.1/23/21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Falina, I.V., Kononenko, N.A., Shkirskaya, S.A. et al. Experimental and Theoretical Study of Influence of Nature of Counterion on Electroosmotic Water Transport in Sulfonated Cation-Exchange Membranes. Membr. Membr. Technol. 4, 281–289 (2022). https://doi.org/10.1134/S2517751622050043
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2517751622050043