Abstract
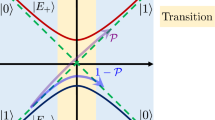
The equation of Levich and Dogonadze describing the rate of electron-transfer processes in the weak-coupling “non-adiabatic” limit is understood in terms of the properties of general adiabatic electron-transfer theory. The cusp diameter describing the continuous changeover of Born–Oppenheimer adiabatic surfaces from donor-like to acceptor-like character is shown to be the critical property controlling reaction rates and intervalence spectra. Their work is presented in the context of general Born–Oppenheimer breakdown phenomena and linked to the overarching cusp catastrophe.
Similar content being viewed by others
References
Levich, V.G. and Dogonadze, R.R., Theory of rediationless electron transitions between ions in solution, Proc. Akad. Nauk SSSR, 1959, vol. 24, pp. 9–13.
Levich, V.G. and Dogonadze, R.R., Adiabatic theory for electron-transfer processes in solution, Proc. Akad. Nauk SSSR, 1960, vol. 133, pp. 156–180.
Levich, V.G. and Dogonadze, R.R., Adiabatic theory of electron-transfer processes in solution, Collect. Czech. Chem. Commun., 1961, vol. 26, p. 193.
Levich, V.G., Present state of the theory of oxidation–reduction in solution (bulk and electrode reactions), in Advances in Electrochemistry and Electrochemical Engineering, vol. 4, Delahaye, P., New York: Interscience Publishers, 1966, p. 249.
Marcus, R.A. and Sutin, N., Electron transfers in chemistry and biology, Biochim. Biophys. Acta, 1985, vol. 811, p. 265.
Kuznetsov, A. and Ulstrup, J., Electron Transfer in Chemistry and Biology: An Introduction to the Theory, Chichester: Wiley, 1998.
Kubo, R. and Toyozawa, Y., Application of the method of generating function to radiative and non- ‡ = 2 q b (Qx ) radiative transitions of a arapped electron in a crystal, Prog. Theor. Phys., 1955, vol. 13, p. 160.
Dirac, P.A.M., The quantum theory of the emission and absorption of radiation, Proc. Royal Soc. London, Ser. A, 1927, vol. 114, p. 243.
Libby, W.F., Theory of electron exchange reactions in aqueous solution, J. Phys. Chem., 1952, vol. 56, p. 863.
Marcus, R.J., Zwolinski, B.J., and Eyring, H., The electron tunnelling hypothesis for electron ex-change reactions, J. Phys. Chem., 1954, vol. 58, p. 432.
Weiss, J., On the theory of electron-transfer processes in aqueous solutions, Proc. R. Soc. London, Ser. A, 1954, vol. 222, p. 128.
Marcus, R.A., On the theory of oxidation-reduction reactions involving electron transfer, Part I, J. Chem. Phys., 1956, vol. 24, p. 966.
Holstein, T., Studies of polaron motion, Part II: The “small” polaron, Annals Physics, 1959, vol. 8, p. 343.
Holstein, T., Studies of polaron motion, Part I: The molecular-crystal model, Annals Physics, 1959, vol. 8, p. 325.
Lax, M., The Franck–Condon principle and its application to crystals, J. Chem. Phys., 1952, vol. 20, p. 1752.
Mielke, S.L., Tawa, G.J., Truhlar, D.G., and Schwenke, D.W., Quantum photochemistry. Accurate quantum scattering calculations for an electronically nonadiabatic reaction, Chem. Phys. Lett., 1995, vol. 234, p. 57.
Polanyi, J.C. and Zewail, A.H., Direct observation of the transition state, Accounts Chem. Res., 1995, vol. 28, p. 119.
Reimers, J.R., The importance of motions that accompany those occurring along the reaction coordinate, Aust. J. Chem., 2015, vol. 68, p. 1202.
Hush, N.S., Adiabatic rate processes at electrodes, J. Chem. Phys., 1958, vol. 28, p. 962.
Hush, N.S., in Soviet electrochemistry: Proceedings of the Fourth Conference on Electrochemistry 1956, Frumkin, A.N., Ed., New York: Consultants Bureau, 1961, p. 99.
Delahay, P., Advances in Electrochemistry and Electrochemical Engineering, vol. 4, Electrochemistry, New York, N.Y.: Interscience, 1966.
Reimers, J.R., McKemmish, L., McKenzie, R.H., and Hush, N.S., Non-adiabatic effects in thermochemistry, spectroscopy and kinetics: the general importance of all three Born–Oppenheimer breakdown corrections, Phys. Chem. Chem. Phys., 2015, vol. 17, p. 24640.
London, F., Zur Quantentheorie der homöopolaren Valenzzahlen, Z. Phys., 1928, vol. 46, p. 455.
London, F., On the theory of non-adiabatic chemical reactions, Z. Phys., 1932, vol. 74, p. 143.
Eyring, H. and Polanyi, M., Concerning simple gas reactions, Z. Phys. Chem. Abt. B, 1931, vol. 12, p. 279.
Evans, M.G. and Polanyi, M., Inertia and driving force of chemical reactions, Trans. Faraday Soc., 1938, vol. 34, p. 11.
Horiuti, J. and Polanyi, M., Outlines of a theory of proton transfer, J. Mol. Catalysis A, 2003, vol. 199, p. 185; Translation of Acta Physicochimica U.R.S.S, 1935, vol., pp. 505–532.
Wall, F.T. and Glockler, G., The double minimum problem applied to the ammonia molecules, J. Chem. Phys., 1937, vol. 5, p. 314.
Hush, N.S., Quantum-mechanical discussion of the gas phase formation of quinonedimethide monomers, J. Polymer Sci., 1953, vol. 11, p. 289.
Reimers, J.R., McKemmish, L., McKenzie, R.H., and Hush, N.S., A unified diabatic description for electron transfer reactions, isomerization reactions, proton transfer reactions, and aromaticity, Phys. Chem. Chem. Phys., 2015, vol. 17, p. 24598.
Schmickler, W., A theory of adiabatic electron-transfer reactions, J. Electroanalyt. Chem. Interfacial Electrochem., 1986, vol. 204, p. 31.
Hush, N.S., Electron transfer in retrospect and prospect 1: Adiabatic electrode processes, J. Electroanal. Chem., 1999, vol. 460, p. 5.
Reimers, J.R., McKemmish, L., McKenzie, R.H., and Hush, N.S., Bond angle variations in XH3 [X = N, P, As, Sb, Bi]: The critical role of Rydberg orbitals exposed using a diabatic state model, Phys. Chem. Chem. Phys., 2015, vol. 17, p. 24618.
McKemmish, L.K., McKenzie, R.H., Hush, N.S., and Reimers, J.R., Quantum entanglement between electronic and vibrational degrees of freedom in molecules, J. Chem. Phys., 2011, vol. 135, p. 244110.
McKemmish, L., McKenzie, R.H., Hush, N.S., and Reimers, J.R., Electron-vibration entanglement in the Born–Oppenheimer description of chemical reactions and spectroscopy, Phys. Chem. Chem. Phys., 2015, vol. 17, p. 24666.
Born, M. and Oppenheimer, R., Zur Quantentheorie der Molekeln, Ann. Phys., 1927, vol. 84, p. 457.
Born, M. and Huang, K., Dynamical Theory of Crystal Lattices, Oxford: Clarendon, 1954.
Ballhausen, C.J. and Hansen, A.E., Electronic spectra, Annu. Rev. Phys. Chem., 1972, vol. 23, p. 15.
Hush, N.S., Electron delocalization, structure and dynamics in mixed-valence systems, NATO Adv. Study Inst. Ser., Ser. C, 1980, vol. 58, p. 151.
Marcus, R.A., Chemical and electrochemical electron-transfer theory, Annu. Rev. Phys. Chem., 1964, vol. 15, p. 155.
Shi, Z. and Boyd, R.J., Charge development at the transition state: a second-order Moeller-Plesset perturbation study of gas-phase SN2 reactions, J. Am. Chem. Soc., 1991, vol. 113, p. 1072.
Williams, A., Effective charge and transition-state structure in solution, in Adv. Phys. Org. Chem., Bethell, D., Academic Press, 1992, p. 1.
Aragonès, A.C., Haworth, N.L., Darwish, N., Ciampi, S., Bloomfield, N.J., Wallace, G.G., Diez-Perez, I., and Coote, M.L., Electrostatic catalysis of a Diels–Alder reaction, Nature, 2016, vol. 531, p. 88.
Shaik, S., Mandal, D., and Ramanan, R., Oriented electric fields as future smart reagents in chemistry, Nat. Chem., 2016, vol. 8, p. 1091.
Wu, Y. and Boxer, S.G., A Critical test of the electrostatic contribution to catalysis with noncanonical amino acids in ketosteroid isomerase, J. Am. Chem. Soc., 2016, vol. 138, p. 11890.
Andrews, D.L., Biological Energy 5: Electron Transfer Theory, Institute of Physics, 2016. biologicalphysics. iop.org/cws/article/lectures/53592.
Hush, N.S., Inequivalent XPS [X-ray photoelectron spectroscopy] binding energies in symmetrical delocalized mixed-valence complexes, Chem. Phys., 1975, vol. 10, p. 361.
Öpik, U. and Pryce, M.H.L., Studies of the Jahn- Teller effect, I: A survey of the static problem, Proc. R. Soc. London, A, 1957, vol. 238, p. 425.
Saunders, P.T., An Introduction to Catastrophe Theory, Cambridge: Cambridge University Press, 1980.
Xu, F., Application of catastrophe theory to the ΔG–to–ΔG relationship in electron transfer reactions, Z. Phys. Chem., 1990, vol. 166, p. 79.
Krokidis, X., Silvi, B., Dezarnaud-Dandine, C., and Sevin, A., Topological study, using a coupled ELF and catastrophe theory technique, of electron transfer in the Li + Cl2 system, New J. Chem., 1998, vol. 22, p. 1341.
Wales, D.J., A microscopic basis for the global appearance of energy landscapes, Science, 2001, vol. 293, p. 2067.
Tishchenko, O., Truhlar, D.G., Ceulemans, A., and Nguyen, M.T., A unified perspective on the hydrogen atom transfer and proton-coupled electron transfer mechanisms in terms of topographic features of the ground and excited potential energy surfaces as exemplified by the reaction between phenol and radicals, J. Am. Chem. Soc., 2008, vol. 130, p. 7000.
Bersuker, I.B., Gorinchoi, N.N., and Polinger, V.Z., On the origin of dynamic instability of molecular systems, Theor. Chim. Acta, 1984, vol. 66, p. 161.
Bersuker, I.B., Pseudo-Jahn—Teller effect–A twostate paradigm in formation, deformation, and transformation of molecular systems and solids, Chem. Rev., 2013, vol. 113, p. 1351.
Thompson, T.C., Truhlar, D.G., and Mead, C.A., On the form of the adiabatic and diabatic representation and the validity of the adiabatic approximation for X[sub 3] Jahn–Teller systems, J. Chem. Phys., 1985, vol. 82, p. 2392.
Kendrick, B.K., Mead, C.A., and Truhlar, D.G., Properties of nonadiabatic couplings and the generalized Born–Oppenheimer approximation, Chem. Phys., 2002, vol. 277, p. 31.
Zhu, C., Jasper, A.W., and Truhlar, D.G., Non- Born–Oppenheimer trajectories with self-consistent decay of mixing, J. Chem. Phys., 2004, vol. 120, p. 5543.
Jasper, A.W. and Truhlar, D.G., Non-Born–Oppenheimer molecular dynamics for conical intersections, avoided crossings, and weak interactions, Adv. Ser. Phys. Chem., 2011, vol. 17, p. 375.
Kolos, W. and Wolniewicz, L., Improved theoretical ground-state energy of the hydrogen molecule, J. Chem. Phys., 1968, vol. 49, p. 404.
Garrett, B.C. and Truhlar, D.G., Nuclear-motion corrections to Born–Oppenheimer barrier heights for chemical reactions, J. Chem. Phys., 1985, vol. 82, p. 4543.
Mielke, S.L., Schwenke, D.W., and Peterson, K.A., Benchmark calculations of the complete configuration-interaction limit of Born–Oppenheimer diagonal corrections to the saddle points of isotopomers of the H + H[sub 2] reaction, J. Chem. Phys., 2005, vol. 122, p. 224313.
Azumi, T. and Matsuzaki, K., What does term vibronic-coupling mean, Photochem. Photobiol., 1977, vol. 25, p. 315.
Handy, N.C., Yamaguchi, Y., and Schaefer Iii, H.F., The diagonal correction to the Born–Oppenheimer approximation: Its effect on the singlet–triplet splitting of CH[sub 2] and other molecular effects, J. Chem. Phys., 1986, vol. 84, p. 4481.
Jensen, J.O. and Yarkony, D.R., On the evaluation of non-Born–Oppenheimer interactions for Born–Oppenheimer wave functions, V: A body fixed frame approach. Applications to isotope effects on equilibrium geometries and the adiabatic correction for the X [sup 1] Sigma [sup + ] state of LiH, J. Chem. Phys., 1988, vol. 89, p. 975.
Valeev, E.F. and Sherrill, C.D., The diagonal Born–Oppenheimer correction beyond the Hartree–Fock approximation, J. Chem. Phys., 2003, vol. 118, p. 3921.
Gauss, J., Tajti, A., Kallay, M., Stanton, J.F., and Szalay, P.G., Analytic calculation of the diagonal Born–Oppenheimer correction within configuration-interaction and coupled-cluster theory, J. Chem. Phys., 2006, vol. 125, p. 144111.
Werner, H.-J., Kallay, M., and Gauss, J. The barrier height of the F + H2 reaction revisited: Coupled-cluster and multireference configuration-interaction benchmark calculations, J. Chem. Phys., 2008, vol. 128, p. 034305/1.
Mohallem, J.R., Coura, T.d.O., Diniz, L.G., De, C.G., Assafrao, D., and Heine, T., Adiabatic corrections to density functional theory energies and wave functions, J. Phys. Chem. A, 2008, vol. 112, p. 8896.
Mohallem, J.R., Semiempirical evaluation of post- Hartree–Fock diagonal-Born–Oppenheimer corrections for organic molecules, J. Chem. Phys., 2008, vol. 128, p. 144113/1.
Tajti, A., Szalay, P.G., and Gauss, J., Perturbative treatment of the electron-correlation contribution to the diagonal Born–Oppenheimer correction, J. Chem. Phys., 2007, vol. 127, p. 014102/1.
Crawford, T.D., Sherrill, C.D., Valeev, E.F., Fermann, J.T., King, R.A., Leininger, M.L., Brown, S.T., Janssen, C.L., Seidl, E.T., Kenny, J.P., and Allen, W.D., PSI3: An open-source ab initio electronic structure package, J. Comput. Chem., 2007, vol. 28, p. 1610.
Harding, M.E. and Klopper, W., Benchmarking the lithium-thiophene complex, Chem. Phys. Chem., 2013, vol. 14, p. 708.
Przybytek, M. and Jeziorski, B., Long-range asymptotic expansion of the diagonal Born–Oppenheimer correction, Chem. Phys., 2012, vol. 401, p. 170.
Pavanello, M., Adamowicz, L., Alijah, A., Zobov, N.F., Mizus, I.I., Polyansky, O.L., Tennyson, J., Szidarovszky, T., and Csaszar, A.G., Calibrationquality adiabatic potential energy surfaces for H3+ and its isotopologues, J. Chem. Phys., 2012, vol. 136, p. 184303/1.
DeYonker, N.J. and Allen, W.D., Taming the lowlying electronic states of FeH, J. Chem. Phys., 2012, vol. 137, p. 234303/1.
Bozkaya, U., Turney, J.M., Yamaguchi, Y., and Schaefer, H.F., III. The lowest-lying electronic singlet and triplet potential energy surfaces for the HNO–NOH system: Energetics, unimolecular rate constants, tunneling and kinetic isotope effects for the isomerization and dissociation reactions, J. Chem. Phys., 2012, vol. 136, p. 164303/1.
Yachmenev, A., Yurchenko, S.N., Ribeyre, T., and Thiel, W., High-level ab initio potential energy surfaces and vibrational energies of H2CS, J. Chem. Phys., 2011, vol. 135, p. 074302/1.
Rao, T.R. and Mahapatra, S., Nuclear motion on the orbitally degenerate electronic ground state of fully deuterated triatomic hydrogen, J. Chem. Phys., 2011, vol. 134, p. 204307/1.
Meier, P., Neff, M., and Rauhut, G., Accurate vibrational frequencies of borane and its isotopologues, J. Chem. Theory Comput., 2011, vol. 7, p. 148.
Lievin, J., Demaison, J., Herman, M., Fayt, A., and Puzzarini, C., Comparison of the experimental, semiexperimental and ab initio equilibrium structures of acetylene: Influence of relativistic effects and of the diagonal Born–Oppenheimer corrections, J. Chem. Phys., 2011, vol. 134, p. 064119/1.
Holka, F., Szalay, P.G., Fremont, J., Rey, M., Peterson, K.A., and Tyuterev, V.G., Accurate ab initio determination of the adiabatic potential energy function and the Born–Oppenheimer breakdown corrections for the electronic ground state of LiH isotopologues, J. Chem. Phys., 2011, vol. 134, p. 094306/1.
Klopper, W., Bachorz, R.A., Tew, D.P., and Hattig, C., Sub-meV accuracy in first-principles computations of the ionization potentials and electron affinities of the atoms H to Ne, Phys. Rev. A: At., Mol., Opt. Phys., 2010, vol. 81, p. 022503/1.
Karton, A. and Martin, J.M.L., Performance of W4 theory for spectroscopic constants and electrical properties of small molecules, J. Chem. Phys., 2010, vol. 133, p. 144102.
Bozkaya, U., Turney, J.M., Yamaguchi, Y., and Schaefer, H.F., III. The barrier height, unimolecular rate constant, and lifetime for the dissociation of HN2, J. Chem. Phys., 2010, vol. 132, p. 064308/1.
Mielke, S.L., Schwenke, D.W., Schatz, G.C., Garrett, B.C., and Peterson, K.A., Functional representation for the Born–Oppenheimer diagonal correction and Born–Huang adiabatic potential energy surfaces for isotopomers of H3, J. Phys. Chem. A, 2009, vol. 113, p. 4479.
Hobson, S.L., Valeev, E.F., Csaszar, A.G., and Stanton, J.F., Is the adiabatic approximation sufficient to account for the post-Born–Oppenheimer effects on molecular electric dipole moments?, Mol. Phys., 2009, vol. 107, p. 1153.
Hirata, S., Miller, E.B., Ohnishi, Y.-y., and Yagi, K., On the validity of the Born–Oppenheimer separation and the accuracy of diagonal corrections in anharmonic molecular vibrations, J. Phys. Chem. A, 2009, vol. 113, p. 12461.
Schwenke, D.W., Beyond the potential energy surface: Ab initio corrections to the Born–Oppenheimer approximation for H2O, J. Phys. Chem. A, 2001, vol. 105, p. 2352.
Newton, M.D. and Sutin, N., Electron transfer reactions in condensed phases, Annu. Rev. Phys. Chem., 1984, vol. 35, p. 437.
Jasper, A.W., Kendrick, B.K., Mead, C.A., and Truhlar, D.G., Non-Born–Oppenheimer chemistry: Potential surfaces, couplings, and dynamics, Adv. Ser. Phys. Chem., 2004, vol. 14, p. 329.
Lee, S.-H., Larsen, A.G., Ohkubo, K., Cai, Z.-L., Reimers, J.R., Fukuzumi, S., and Crossley, M.J., Long-lived long-distance photochemically induced spin-polarized charge separation in β,β'-pyrrolic fused ferrocene-porphyrin-fullerene systems, Chem. Sci., 2012, vol. 3, p. 257.
Yin, S., Li, L., Yang, Y., and Reimers, J.R., Challenges for the accurate simulation of anisotropic charge mobilities through organic molecular crystals: The β phase of mer-Tris(8-hydroxyquinolinato)aluminum(III) (Alq3) crystal, J. Phys. Chem. C, 2012, vol. 116, p. 14826.
Norton, J.E. and Brédas, J.L., Polarization energies in oligoacene semiconductor crystals, J. Am. Chem. Soc., 2008, vol. 130, p. 12377.
Hush, N.S., Intervalence-transfer absorption, II: Theoretical considerations and spectroscopic data, Prog. Inorg. Chem., 1967, vol. 8, p. 391.
Kjaer, A.M. and Ulstrup, J., Solvent bandwidth dependence and band asymmetry features of chargetransfer transitions in N-pyridinium phenolates, J. Am. Chem. Soc., 1987, vol. 109, p. 1934.
Reimers, J.R. and Hush, N.S., Electronic properties of transition-metal complexes determined from electro- absorption spectroscopy II mono-nuclear complexes of ruthenium(II), J. Phys. Chem., 1991, vol. 95, p. 9773.
Reimers, J.R. and Hush, N.S., The effects of couplings to symmetric and antisymmetric modes and minor asymmetries on the spectral properties of mixed-valence and related charge-transfer systems, Chem. Phys., 1996, vol. 208, p. 177.
Reimers, J.R. and Hush, N.S., Hamiltonian operators including both symmetric and antisymmetric vibrational modes for vibronic-coupling and intervalence charge-transfer applications, Chem. Phys., 2004, vol. 299, p. 79.
Lockhart, D.J. and Boxer, S.G., Electric field modulation of the fluorescence spectrum from rhodobacter sphaeroides reaction centers, Chem. Phys. Lett., 1988, vol. 144, p. 243.
Boxer, S.G., Goldstein, R.A., Lockhart, D.J., Middendorf, T.R., and Takiff, L., Excited states, electrontransfer reactions, and intermediates in bacterial photosynthetic reaction centers, J. Phys. Chem., 1989, vol. 93, p. 8280.
Fried, S.D., Bagchi, S., and Boxer, S.G., Extreme electric fields power catalysis in the active site of ketosteroid isomerase, Science, 2014, vol. 346, p. 1510.
Oh, D.H., Sano, M., and Boxer, S.G., Electroabsorption (Stark effect) spectroscopy of mono- and biruthenium charge-transfer complexes: measurements of changes in dipole moments and other electrooptic properties, J. Am. Chem. Soc., 1991, vol. 113, p. 6880.
Cave, R.J. and Newton, M.D., Generalization of the Mulliken-Hush treatment for the calculation of electron transfer matrix elements, Chem. Phys. Lett., 1996, vol. 249, p. 15.
Hush, N.S., Discussion of “Electrode reactons of organic compounds: General Introduction” by R.A. Marcus, Discus. Faraday Soc., 1968, vol. 45, p. 52.
Hammond, G.S., A correlation of reaction rates, J. Am. Chem. Soc., 1955, vol. 77, p. 334.
Leffler, J.E., Parameters for the description of transition states, Science, 1953, vol. 117, p. 340.
Toro-Labbe, A., Characterization of chemical reactions from the profiles of energy, chemical potential, and hardness, J. Phys. Chem. A, 1999, vol. 103, p. 4398.
Toro-Labbe, A., Gutierrez-Oliva, S., Murray, J.S., and Politzer, P., A new perspective on chemical and physical processes: The reaction force, Mol. Phys., 2007, vol. 105, p. 2619.
Toro-Labbe, A., Gutierrez-Oliva, S., Murray, J.S., and Politzer, P., The reaction force and the transition region of a reaction, J. Mol. Model., 2009, vol. 15, p. 707.
Politzer, P., Reimers, J.R., Murray, J.S., and Toro-Labbe, A., Reaction force and its link to diabatic analysis: A unifying approach to analyzing chemical reactions, J. Phys. Chem. Lett., 2010, vol. 1, p. 2858.
Kanchanawong, O., Dahlbom, M.G., Treynor, T.P., Reimers, J.R., Hush, N.S., and Boxer, S.G., Charge delocalization in the special pair radical cation of mutant reaction centers of rhodobacter sphaeroides from stark spectra and non-adiabatic spectral simulations, J. Phys. Chem. B, 2006, vol. 110, p. 18688.
Reimers, J.R. and Hush, N.S., Understanding the observed Stark spectra, midpoint potential versus degree of charge localization, and intervalence transition energies of the special-pair radical cation of Rhodobacter sphaeroides and its mutant strains, J. Am. Chem. Soc., 2004, vol. 126, p. 4132.
Reimers, J.R. and Hush, N.S., Electric field perturbation of electronic (vibronic) absorption envelopes: Application to characterization of mixed-valence states, in Mixed Valence Systems: Applications in Chemistry, Physics, and Biology, Prassides, K., Dordrecht: Kluwer Acad. Publishers, 1991, p. 29.
Franzen, S., Lao, K., and Boxer, S.G., Electric field effects on kinetics of electron transfer reactions: Connection between experiment and theory, Chem. Phys. Lett., 1992, vol. 197, p. 380.
Landau, L.D., Z. Phys. Sowjetunion, 1932, vol. 2, p. 46.
Landau, L.D., Z. Phys. Sowjetunion, 1932, vol. 1, p. 88.
London, F., Ueber den Mechanismus der homöopolaren Bindung, in Probleme der Modernen Physik, Sommerfeld, A. and Debye, P., Leipzig: Hirsel, 1928, p. 104.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is the authors’ contribution to the special issue of Russian Journal of Electrochemistry dedicated to the 100th anniversary of the birth of the outstanding Soviet electrochemist Veniamin G. Levich.
Published in Russian in Elektrokhimiya, 2017, Vol. 53, No. 9, pp. 1169–1182.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Reimers, J.R., Hush, N.S. The critical role of the transition-state cusp diameter in understanding adiabatic and non-adiabatic electron transfer. Russ J Electrochem 53, 1042–1053 (2017). https://doi.org/10.1134/S1023193517090105
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193517090105