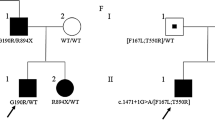
Abstract
Thomsen’s (TM) and Becker’s (BM) myotonias are nondystrophic myotonias. At present, 150 mutations in the CLCN1 gene, which results in the development of TM and BM, have been described. The c.2680C>T (p.Arg894*) is the most common mutation. In the Northern Scandinavian countries, the population frequency of this mutation is 0.87%, while in the Russian Federation, it is equal to 1.2% (this study). Based on the results of a molecular-genetic analysis of CLCN1 gene in patients with nondystrophic myotonias, the calculated frequency of TM and BM in Russia is 1: 8165 and 1: 710, respectively. We have conducted haplotype analysis using microsatellite markers and intragene SNP, which has shown that the prevalence of p.Arg894* mutation in Russia results from the founder effect, and the time of its scattering is 3680 ± 1240 years.
Similar content being viewed by others
References
Meyer-Kleine, C., Steinmeyer, K., Riecker, K., et al., Spectrum of mutations in the major human skeletal muscle chloride channel gene (CLCN1) leading to myotonia, Am. J. Hum. Genet., 1995, vol. 57, no. 6, pp. 1325–1334.
Papponen, H., Toppinen, T., Baumann, P., et al., Founder mutations and the high prevalence of myotonia congenita in northern Finland, J. Neurol., 1999, vol. 53, no. 2, pp. 297–302.
Sun, C., Tranebjaerg, L., Torbergsen, T., et al., Spectrum of CLCN1 mutations in patients with myotonia congenita in northern Scandinavia, Eur. J. Hum. Genet., 2001, vol. 9, no. 12, pp. 903–909.
Trip, J., Drost, G., Verbove, D.J., et al., In tandem analysis of CLCN1 and SCN4A greatly enhances mutation detection in families with non-dystrophic myotonia, Eur. J. Hum. Genet., 2008, vol. 16, no. 8, pp. 921–929.
Mankodi, A., Myotonic disorders, Neurol. India, 2008, vol. 56, no. 3, pp. 298–304.
George, A.L., Crackower, M.A., Abdalla, J.A., et al., Molecular basis of Thomsen’s disease (autosomal dominant myotonia congenita), Nat. Genet., 1993, vol. 3, no. 4, pp. 305–310.
Koty, P.P., Pegoraro, E., Hobson, G., et al., Myotonia and the muscle chloride channel: dominant mutations show variable penetrance and founder effect, Neurology, 1996, vol. 47, no. 4, pp. 963–968.
Koch, M.C., Steinmeyer, K., Lorenz, C., et al., The skeletal muscle chloride channel in dominant and recessive human myotonia, Science, 1992, vol. 257, no. 5071, pp. 797–800.
Ivanova, E.A., Dadali, E.L., Fedotov, V.P., et al., The spectrum of CLCN1 gene mutations in patients with nondystrophic Thomsen’s and Becker’s myotonias, Russ. J. Genet., 2012, vol. 49, no. 9, pp. 952–961.
Bengtsson, B.O. and Thomson, G., Measuring the strength of associations between HLA antigens and diseases, Tissue Antigens, 1981, vol. 18, pp. 356–363.
Bliznets, E.A., Zinchenko, R.A., Tverskaya, S.M., et al., Population frequency and the age of mutation in c.807+5G>A, causing autosomal recessive osteopetrosis in Chuvashia, Med. Genet., 2006, vol. 5, no. 9, pp. 9–14.
Labuda, D., Zietkiewicz, E., and Labuda, M., The genetic clock and the age of the founder effect in growing populations: A lesson from French Canadians and Ashkenazim, Am. J. Hum. Genet., 1997, vol. 61, pp. 768–771.
Risch, N., de Leon, D., Ozelius, L., et al., Genetic analysis of idiopathic torsion dystonia in Ashkenazi Jews and their recent descent from a small founder population, Nat. Genet., 1995, vol. 9, no. 2, pp. 152–159.
Colombo, R., Age estimate of the N370S mutation causing Gaucher disease in Ashkenazi Jews and European populations: a reappraisal of haplotype data, Am. J. Hum. Genet., 2000, vol. 66, pp. 692–697.
Durst, R., Colombo, R., Shpitzen, S., et al., Recent origin and spread of a common Lithuanian mutation, G197del LDLR, causing familial hypercholesterolemia: positive selection is not always necessary to account for disease incidence among Ashkenazi Jews, Am. J. Hum. Genet., 2001, vol. 68, pp. 1172–1188.
Zielonka, D., Jurkat-Rott, K., Stachowiak, P., et al., A Becker myotonia patient with compound heterozygosity for CLCN1 mutations and Prinzmetal angina pectoris, Neuromuscular Disord., vol. 22, no. 4, pp. 355–360.
Dun, M., Colding-Jørgensen, E., and Grunnet, M., Difference in allelic expression of the CLCN1 gene and the possible influence on the myotonia congenita phenotype, Eur. J. Hum. Genet., 2004, vol. 12, no. 9, pp. 738–743.
Sangiuolo, F., Botta, A., Mesoraca, A., et al., Identification of five new mutations and three novel polymorphisms in the muscle chloride channel gene (CLCN1) in 20 Italian patients with dominant and recessive myotonia congenita, Hum. Mutat., 1998, vol. 11, no. 4, p. 331.
Dupré, N., Chrestian, N., Bouchard, J.P., et al., Clinical, electrophysiologic, and genetic study of non-dystrophic myotonia in French-Canadians, Neuromuscular Disord., 2009, vol. 19, no. 5, pp. 330–334.
Mazón, M.J., Barros, F., de la Peña, P., et al., Screening for mutations in Spanish families with myotonia: functional analysis of novel mutations in CLCN1 gene, Neuromuscular Disord., 2012, vol. 22, no. 3, pp. 303–304.
Lin, M.J., You, T.H., Pan, H., et al., Functional characterization of CLCN1 mutations in Taiwanese patients with myotonia congenita via heterologous expression, Biochem. Biophys. Res. Commun., 2006, vol. 351, no. 4, pp. 1043–1047.
Guzeva, V.I., Rukovodstvo po detskoi nevrologii (Guide to Child Neurology), St. Petersburg: Med. Inform. Agenstvo, 2009.
Gudz’-Markov, A.V., Indoevropeiskaya istoriya Evrazii: Proiskhozhdenie slavyanskogo mira, (Indo-European History of Eurasia: Origin of Slavic World), Moscow: Rikel, 1995.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.A. Ivanova, A.V. Polyakov, 2013, published in Genetika, 2013, Vol. 49, No. 12, pp. 1407–1415.
Rights and permissions
About this article
Cite this article
Ivanova, E.A., Polyakov, A.V. Frequency and causes of prevalence of p.Arg894* mutation in CLCN1 gene responsible for development of thomsen’s and becker’s myotonias in russian population. Russ J Genet 49, 1227–1235 (2013). https://doi.org/10.1134/S1022795413090044
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795413090044