Abstract
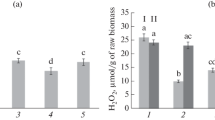
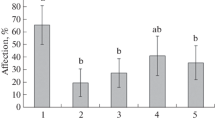
The effect of Bacillus subtilis bacteria in combination with salicylic (SA) and jasmonic (JA) acids on changes in the proteome of potato leaves during infection with Phytophthora infestans (Mont.) de Bary and moisture deficit was studied. Plants grown from microtubers of the Rannyaya Roza variety were sprayed with a suspension of B. subtilis (108 cells/mL) and a mixture of bacteria with SA (10–6 M), JA (10–7 M), and SA + JA. The plants were infected with P. infestans (105 spores/mL) 3 days after treatment and cultivated under conditions of artificial soil drought by reducing watering. When the soil moisture reached 40 ± 5% of the total water capacity (7 days after infection), the plants were fixed in liquid nitrogen to isolate proteins and analyze them by two-dimensional electrophoresis and mass spectrometry. A decrease in the degree of infestation with P. infestans was shown when potato leaves were treated with B. subtilis in combination with SA and JA. The leaf proteome shows differences in the content of 14 proteins in the pI range from 4.0 to 9.0 with molecular masses from 30 to 125 kDa. The most significant changes in the spectrum of plant proteins were found in healthy plants treated with B. subtilis and in infected plants in the combination of B. subtilis with JA. Qualitative and quantitative changes were observed for proteins involved in the processes of respiration and hypersensitivity reactions (HSR), energy metabolism, synthesis of secondary metabolites, and protective proteins that affect plant resistance to abiotic and biotic stress. Proteomic analysis has identified important proteins involved in the mechanism of potato responses to treatment with B. subtilis and infection with P. infestans, although the exact functions of the identified proteins and their potential impact on potato plant resistance to P. infestans and moisture deficit remain to be elucidated.



Similar content being viewed by others
REFERENCES
Verma, P., Yadav, A.N., Kumar, V., Singh, D.P., and Saxena, A.K., Beneficial plant-microbes interactions: biodiversity of microbes from diverse extreme environments and its impact for crop improvement, in Plant-Microbe Interactions in Agro-Ecological Perspectives, Singh, D.P., Singh, H.B., and Prabha, R., Eds., Singapore: Springer-Verlag, 2017, p. 543.
Burton, W.G., Challenges for stress physiology in potato, Am. Potato J., 1981, vol. 58, p. 3.
Coleman-Derr, D. and Tringe, S.G., Building the crops of tomorrow: advantages of symbiont-based approaches to improving abiotic stress tolerance, Front. Microbiol., 2014, vol. 5, p. 283. https://doi.org/10.3389/fmicb.2014.00283
Berg, G., Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture, Appl. Microbiol. Biotechnol., 2009, vol. 84, p. 11. https://doi.org/10.1007/s00253-009-2092-7
Burkhanova, G.F., Veselova, S.V., Sorokan, A.V., Blagova, D.K., Nuzhnaya, T.V., and Maksimov, I.V., Strains of Bacillus ssp. regulate wheat resistance to Septoria nodorum Berk, Appl. Biochem. Microbiol., 2017, vol. 53, p. 346. https://doi.org/10.18699/VJ19.561
Chanda, B., Xia, Y., Mandal, M.K., Sekine, K.T., Gao, Q.M., and Selote, D., Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants, Nat. Genet., 2011, vol. 43, p. 421. https://doi.org/10.1038/ng.798
Kuznetsova, M.A., Kozlovskii, B.E., Beketova, M.P., Sokolova, E.A., Malyuchenko, O.P., Alekseev, Ya.I., Rogozina, E.V., and Khavkin, E.E., Phytopathological and molecular characteristics of Phytophthora infestans isolates collected from resistant and sensitive potato genotypes, Mikol. Fitopatol., 2016, vol. 50, p. 175.
Batool, T., Ali, S., Seleiman, M.F., Naveed, N.H., Ali, A., Ahmed, K., Abid, M., Rizwan, M., Shahid, M.R., Alotaibi, M., Al-Ashkar, I., and Mubushar, M., Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities, Sci. Rep., 2020, vol. 10, p. 1. https://doi.org/10.1038/s41598-020-73489-z
Vasyukova, N.I., Chalenko, G.I., Gerasimova, N.G., Valueva, T.A., and Ozeretskovskaya, O.L., Activation of elicitor defensive properties by systemic signal molecules during the interaction between potato and the late blight agent, Appl. Biochem. Microbiol., 2008, vol. 44, p. 213. https://doi.org/10.1007/s10438-008-2015-x
Vincent, D., Wheatley, M., and Cramer, G., Optimization of protein extraction and solubilization for mature grape berry, Electrophoresis, 2006, vol. 27, p. 1853. https://doi.org/10.1002/elps.200500698
Fedina, E.O., Karimova, F.G., Tarchevsky, I.A., Toropygin, I.Y., and Khripach, V.A., Effect of epibrassinolide on tyrosine phosphorylation of the Calvin cycle enzymes, Russ. J. Plant Phys., 2008, vol. 55, p. 193. https://doi.org/10.1007/s11183-008-2005-0
Suckau, D., Resemann, A., Schuerenberg, M., Hufnagel, P., Franzen, J., and Holle, A., A novel MALDI LIFT-TOF/TOF mass spectrometer for proteomics, Anal. Bioanal. Chem., 2003, vol. 376, p. 952. https://doi.org/10.1007/s00216-003-2057-0
Perkins, D.N., Pappin, D.J., Creasy, D.M., and Cottrell, J.S., Probability-based protein identification by searching sequence databases using mass spectrometry data, Electrophoresis, 1999, vol. 20, p. 3551. https://doi.org/10.1002/(SICI)1522-2683(19991201)20
Yarullina, L.G., Kasimova, R.I., Ibragimov, R.I., Akhatova, A.R., Umarov, I.A., and Maksimov, I.V., Qualitative and quantitative changes of potato tuber proteome under the influence of signal molecules and infection with Phytophthora infestans, Appl. Biochem. Microbiol., 2016, vol. 52, p. 71. https://doi.org/10.1134/S0003683816010154
Maksimov, I.V., Sorokan, A.V., Chereoanova, E.A., Surina, O.B., Troshina, N.B., and Yarullina, L.G., Effects of salicylic and jasmonic acids on the components of pro/antioxidant system in potato plants infected with late blight, Russ. J. Plant Phys., 2011, vol. 52, p. 299. https://doi.org/10.1134/S1021443711010109
Gervais, T., Creelman, A., Li, X., Bizimungu, B., De Koeyer, D., and Dahal, K., Potato response to drought stress: physiological and growth basis, Front. Plant Sci., 2021, vol. 12, p. 1360. https://doi.org/10.3389/fpls.2021.698060
Máthé, C., Garda, T., Freytag, C., and M-Hamvas, M., The role of serine-threonine protein phosphatase PP2A in plant oxidative stress signaling—facts and hypotheses, Int. J. Mol. Sci., 2019, vol. 20, art. ID 3028. https://doi.org/10.3390/ijms20123028
Belhaj, K., Lin, B., and Mauch, F., The chloroplast protein RPH1 plays a role in the immune response of Arabidopsis to Phytophthora brassicae, Plant J., 2009, vol. 58, p. 278. https://doi.org/10.1111/j.1365-313X.2008.03779.x
Zadražnik, T., Moen, A., and Šuštar-Vozlič, J., Chloroplast proteins involved in drought stress response in selected cultivars of common bean (Phaseolus vulgaris L.), 3Biotech, 2019, vol. 9, art. ID 331. https://doi.org/10.1007/s13205-019-1862-x
Li, D., Feng, Y., Lu, Q., Yan, D., Liu, Z., and Zhang, X., Comparative proteomic analysis of plant–pathogen interactions in resistant and susceptible poplar ecotypes infected with Botryosphaeria dothidea, Phytopathology, 2019, vol. 109, p. 2009. https://doi.org/10.1094/PHYTO-12-18-0452-R
Ali, A., Goswami, S., Kumar, R.R., Singh, K., Singh, J.P., Kumar, A., Sakhrey, A., Ra, G.K., and Praveen, S., Wheat oxygen evolving enhancer protein: identification and characterization of Mn-binding metalloprotein of photosynthetic pathway involved in regulating photosytem II integrity and network of antioxidant enzymes under heat stress, Int. J. Curr. Microbiol. Appl. Sci., 2018, vol. 7, p. 177. https://doi.org/10.20546/ijcmas.2018.702.02
Moreno, J.I., Martın, R., and Castresana, C., Arabidopsis SHMT1, a serine hydroxymethyltransferase that functions in the photorespiratory pathway influences resistance to biotic and abiotic stress, Plant J., 2005, vol. 41, p. 451. https://doi.org/10.1111/j.1365-313X.2004.02311.x
Fang, C., Zhang, P., and Li, L., Serine hydroxymethyltransferase localised in the endoplasmic reticulum plays a role in scavenging H2O2 to enhance rice chilling tolerance, BMC Plant Biol., 2020, vol. 20, art. ID 236. https://doi.org/10.1186/s12870-020-02446-9
Kandoth, P.K., Liu, S., Prenger, E., Ludwig, A., Lakhssassi, N., Heinz, R., Zhou, Z., Howland, A., Gunther, J., Eidson, S., Dhroso, A., LaFayette, P., Tucker, D., Johnson, S., Anderson, J., Alaswad, A., et al., Systematic mutagenesis of serine hydroxymethyltransferase reveals an essential role in nematode resistance, Plant Physiol., 2017, vol. 175, p. 1370. https://doi.org/10.1104/pp.17.00553
Durian, G., Rahikainen, M., Alegre, S., Brosché, M., and Kangasjärvi, S., Protein phosphatase 2A in the regulatory network underlying biotic stress resistance in plants, Front. Plant Sci., 2016, vol. 7, art. ID 812. https://doi.org/10.3389/fpls.2016.00812
López-Calcagno, P.E., Fisk, S., Brown, K.L., Bull, S.E., South, P.F., and Raines, C.A., Overexpressing the H‑protein of the glycine cleavage system increases biomass yield in glasshouse and field-grown transgenic tobacco plants, Plant Biotechnol. J., 2019, vol. 17, p. 141. https://doi.org/10.1111/pbi.12953
Zhang, X., Li, M., Xu, Y., Ren, J., and Zeng, A.-P., Quantitative study of H protein lipoylation of the glycine cleavage system and a strategy to increase its activity by co-expression of LplA, J. Biol. Eng., 2019, vol. 13, art. ID 32. https://doi.org/10.1186/s13036-019-0164-5
Engel, N., van den Daele, K., Kolukisaoglu, U., Morgenthal, K., Weckwerth, W., Parnik, T., Keerberg, O., and Bauwe, H., Deletion of glycine decarboxylase in Arabidopsis is lethal under nonphotorespiratory conditions, Plant Physiol., 2007, vol. 144, p. 1328. https://doi.org/10.1104/pp.107.099317
Palmieri, M.C., Lindermayr, C., Bauwe, H., Steinhauser, C., and Durner, J., Regulation of plant glycine decarboxylase by S-nitrosylation and glutathionylation, Plant Physiol., 2010, vol. 152, p. 1514. https://doi.org/10.1104/pp.109.152579
Merewitz, E.B., Gianfagna, T., and Huang, B., Protein accumulation in leaves and roots associated with improved drought tolerance in creeping bentgrass expressing an ipt gene for cytokinin synthesis, J. Exp. Bot., 2011, vol. 62, p. 5311. https://doi.org/10.1093/jxb/err166
Pu, Q., Liang, J., Shen, Q., Fu, J., Pu, Z., Liu, J., Wang, X., and Wang, Q., A wheat β-patchoulene synthase confers resistance against herbivory in transgenic Arabidopsis, Genes, 2019, vol. 10, art. ID 441. https://doi.org/10.3390/genes10060441
Liu, G., Yang, M., and Fu, J., Identification and characterization of two sesquiterpene synthase genes involved in volatile-mediated defense in tea plant (Camellia sinensis), Plant Physiol. Biochem., 2020, vol. 155, p. 650. https://doi.org/10.1016/j.plaphy.2020.08.004
Dwivedi, V., Rao, S., Bomzan, D.P., Kumar, S.R., Shanmugam, P.V., Olsson, S.B., and Nagegowda, D.A., Functional characterization of a defense-responsive bulnesol/elemol synthase from potato, Physiol. Plant., 2021, vol. 171, p. 7. https://doi.org/10.1111/ppl.13199
Singh, B. and Sharma, R.A., Plant terpenes: defense responses, phylogenetic analysis, regulation and clinical applications, 3Biotech, 2015, vol. 5, p. 129. https://doi.org/10.1007/s13205-014-0220-2
Ye, J., Mao, D., Cheng, S., Zhang, X., Tan, J., Zheng, J., and Xu, F., Comparative transcriptome analysis reveals the potential stimulatory mechanism of terpene trilactone biosynthesis by exogenous salicylic acid in Ginkgo biloba, Ind. Crops Prod., 2020, vol. 145, art. ID 112104. https://doi.org/10.1016/j.indcrop.2020.112104
Rixen, S., Havemeyer, A., Tyl-Bielicka, A., Pysniak, K., Gajewska, M., Kulecka, M., Ostrowski, J., Mikula, M., and Clement, B., Mitochondrial amidoxime-reducing component 2 (MARC2) has a significant role in N-reductive activity and energy metabolism, J. Biol. Chem., 2019, vol. 294, p. 17593. https://doi.org/10.1074/jbc.RA119.007606
Plitzko, B., Havemeyer, A., Kunze, T., and Clement, B., The pivotal role of the mitochondrial amidoxime reducing component 2 in protecting human cells against apoptotic effects of the base analog N 6-hydroxylaminopurine, Cell Biol., 2015, vol. 290, p. 10126. https://doi.org/10.1074/jbc.M115.640052
Zhao, Q. and Liu, C., Chloroplast chaperonin: an intricate protein folding machine for photosynthesis, Front. Mol. Biosci., 2018, vol. 4, art. ID 98. https://doi.org/10.3389/fmolb.2017.00098
Tsai, Y.C., Mueller-Cajar, O., Saschenbrecker, S., Hartl, F.U., and Hayer-Hartl, M., Chaperonin cofactors, Cpn10 and Cpn20, of green algae and plants function as hetero-oligomeric ring complexes, J. Biol. Chem., 2012, vol. 287, p. 20471. https://doi.org/10.1074/jbc.M112.365411
Ranford, J.C. and Henderson, B., Chaperonins in disease: mechanisms, models, and treatments, Mol. Pathol., 2002, vol. 55, p. 209. https://doi.org/10.1136/mp.55.4.209
Funding
The work was partly carried out on the topic of the State Assignment (state registration number AAAA-A21-121011990120-7), with the financial support of the Russian Foundation for Basic Research and the Belarusian Republican Foundation for Basic Research within the framework of scientific project no. 20-516-00005, using the equipment of the Center for Collective using the “Human Proteome” (IBMC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any research involving humans or animals as research objects.
Additional information
Translated by M. Shulskaya
Abbreviations: JA—jasmonic acid; ISR—induced systemic resistance; SA—salicylic acid; SOD—superoxide dismutase; PGSB—plant growth stimulating bacteria; SAR—systemic acquired resistance; GCS—glycine cleavage system; GDC—glycine dehydrogenase (decarboxylating); mARK—mitochondrial amidoxime reducing component; OEEP—oxygen-evolving enhancer protein; RBOH—respiratory burst oxidase homologs; SHMT—serine hydroxymethyltransferase.
Rights and permissions
About this article
Cite this article
Yarullina, L.G., Tsvetkov, V.O., Khabibullina, V.O. et al. Impact of Bacillus subtilis Bacteria in Combination with Salicylic and Jasmonic Acids on Changing the Proteome of Potato Leaves when Infected by Phytophthora infestans (Mont.) De Bary and with a Moisture Deficit. Russ J Plant Physiol 69, 81 (2022). https://doi.org/10.1134/S1021443722040215
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443722040215